Abstract
The thermal behaviour of two crocidolite asbestos samples was characterized. Infrared spectroscopy (FT-IR), powder X-ray diffraction, scanning electron microscopy and thermal analysis (DTA and TG) with evolved gas analysis were carried out on samples of crocidolite asbestos as received and after heating in order to observe their structural changes and dehydroxylation process. The results show that the dehydroxylation process of crocidolite asbestos occurs at different temperatures and is in the range 400–650 °C. This temperature is dependent on the origin of asbestos samples and may be associated with an increased amount of magnesium in the structure of asbestos. For the sample which comes from the Republic of South Africa, the dehydroxylation process occurs at lower temperature in contrast to Russian crocidolite asbestos sample from cement–asbestos material. However, this temperature range is lower than those reported by some authors, who state that the decomposition temperature of crocidolite should be at least 900 °C. This finding may have a significant influence on thermal utilization of asbestos materials, e.g. cement–asbestos, because the temperature of the heat treatment in the utilization process should be as low as possible.
Similar content being viewed by others
Introduction
Asbestos is a commercial term applied to a group of naturally occurring silicate minerals that have a fibrous crystal structure. Among the six known asbestos minerals, only three have been used on a large industrial scale: chrysotile Mg3[Si2O5](OH)4, crocidolite Na2Fe3Fe2[(OH)Si4O11]2 and amosite (Fe,Mg)7[(OH)Si4O11]2. Asbestos has advantageous physical and chemical properties such as high tensile strength, abrasion resistance, heat resistance and chemical resistance. Therefore, it was used for numerous materials, especially in the building materials industry (cement–asbestos products that in Poland are known under the name “eternit”) [1–4].
The asbestos era ended in many countries in the late twentieth century when carcinogenic properties associated with the penetration of the respirable asbestos fibres into the human respiratory system were discovered [5–7]. In the 1970s of the twentieth century, the Polish industry imported about 100,000 tons of asbestos annually, of which over 90 % was chrysotile. In the 1980s, consumption of asbestos dropped and in the 1991 there was only 30,000 tons of it. It was only chrysotile asbestos because in 1985 the use of crocidolite asbestos in Poland was discontinued due to its extreme toxicity. Chrysotile asbestos was banned in Poland in the 1997 [8]. Currently, asbestos minerals are considered as hazardous material. Nowadays, in Poland there is only one way to deal with this hazardous waste, which is storing in controlled landfill sites [9]. This does not completely solve the asbestos problem, as the asbestos fibre structure is not entirely destroyed, and these wastes are only isolated from the human environment.
One of the possible methods of asbestos disposal may be heat treatment. Asbestos minerals belong to a group of hydrated silicates and the thermal treatment of them results in dehydroxylation at temperatures depending on the type of asbestos minerals. Thermal decomposition of asbestos minerals should generally take place according to three stages [10, 11]. The first stage is associated with the loss of adsorbed water. The next step is the dehydroxylation process, which is connected with the removal of structural OH groups from the structure of asbestos minerals. The last stage is responsible for the crystallization of amorphous material leading to the formation of new phases. This leads to a change in the crystalline structure and the formation of new phases without a dangerous, fibrous structure.
Thermal decomposition of chrysotile asbestos is fairly well understood and has been described in the technical literature [11–24]. A summary of the thermal transformations of chrysotile asbestos, which was approximately 90 % of world production, is represented by the following reaction path [25]:
The dehydroxylation process of chrysotile asbestos occurs at around 600–750 °C (with the maximum rate above 700 °C) followed by subsequent recrystallization at high temperatures (at above 800 °C), leading to the formation of forsterite and enstatite.
There is much less literature describing the thermal decomposition of the crocidolite asbestos type, and its thermal behaviour is still poorly understood. Generally, depending on the authors, thermal decomposition of crocidolite asbestos takes place in a wide temperature range from 400 to 900 °C, which is reported in the literature concerning the general problem of asbestos [26–28]. There are only a few papers that describe the thermal decomposition of crocidolite asbestos. Their interpretation is not so clear as in the case of chrysotile thermal decomposition. In [29], the authors showed the DTA curves for crocidolite specimens. They observed an exothermic peak at ~400 °C, an endothermic peak at 900 °C (which is explained by the dehydroxylation process) and a small exothermic peak at 920–960 °C (crystallization). The authors explained the thermal effect at ~400 °C as an oxidation process. During this process, the conversion of Fe2+ to Fe3+ occurs. In [30], authors show DTA curves of crocidolite asbestos recorded in oxygen and argon atmosphere. In oxygen, fresh crocidolite has an exotherm between 400 and 450 °C and a marked endotherm just above 900 °C, followed by an exotherm. In argon atmosphere, the dehydroxylation endotherm takes place between 610 and 680 °C and the decomposition occurs between 800 and 880 °C. On the other hand, the authors in [31] did not observe any mass loss of crocidolite asbestos in oxygen atmosphere above ~700 °C, and the total mass loss recorded for this temperature was 2 mass%. However, on the DTA curve, they observed a strong endothermic peak (T max ~ 930 °C) without a change in the mass. SEM observations show a fibrous-like structure that was melt-bonded with one another. The authors in [32] showed thermal analysis curves of crocidolite asbestos and stated that the major mass loss occurs before 700 °C. In turn, the authors in [33] stated that the dehydroxylation temperature of crocidolite asbestos in an inert atmosphere is 520 °C. This temperature was connected with the maximum rate of mass loss due to the water release. On the other hand, while crocidolite is dynamically heated in air at 400–600 °C, the hydrogen ions and electrons are lost to give an oxy-amphibole (Na2Fe4FeSi8O24; oxycrocidolite) and the crocidolite lost the water in the temperature range between 400 and 500 °C [34]. According to Addison et al. [35], the oxidation of crocidolite at 450 °C involves simultaneous oxidation of ferrous and hydroxyl ions, and to a good approximation follows the equation:
with a secondary surface reaction:
The reaction takes place on the surface and is maintained by the migration of electrons and protons through the crystal. This process, by which a ferrous ion is produced on the surface, is analogous to positive hole conduction and involves transfer of an electron from one ferrous ion to a neighbouring ferric ion, and this process is continued until there is a ferrous ion on the surface [35].
In the case of static heating, this process takes place at 300–450 °C. At 600–950 °C, the oxycrocidolite is decomposable endothermically, and most of the Fe2+ is oxidized giving acmite (NaFeSi2O6), hematite (Fe2O3), cristobalite (SiO2) and a spinel (FeFe2O4). At 975–1,000 °C, acmite decomposes and the melting begins [34]. Moreover, Hodgson [30] suggests that the presence of magnesium exerts a blocking effect on the decomposition of crocidolite and at higher magnesium concentrations the final decomposition process is shifted to higher temperatures.
Knowledge of a more precise temperature range of the crocidolite dehydroxylation process is very important with respect to thermal utilization of asbestos-containing materials and may help in reducing the treatment temperature, as this way of recycling asbestos and asbestos-containing materials is one of the most popular methods that has been proposed in the technical literature. This is particularly important in the case of thermal utilization of materials manufactured from the crocidolite asbestos and cement–asbestos products, containing crocidolite in addition to the chrysotile asbestos. The aim of the thermal utilization is decomposition of asbestos minerals, which produces safe products for use. From an economic point of view (expenditure of energy), it is desirable that the temperature of the heat treatment in the utilization process is as low as possible.
The aim of the study was to determine a more precise range of the crocidolite asbestos dehydroxylation temperature and thereby to set a minimum calcination temperature for its further thermal utilization.
Experimental
In the present study, two different types of crocidolite asbestos were examined. One of the samples was crocidolite from the Republic of South Africa (marked as K1). In the research, we also used crocidolite asbestos that was separated from a corrugated asbestos–cement (a–c) slate (marked as K2). This slate was produced a few decades ago, and crocidolite (besides chrysotile) asbestos from former USRR (Soviet Union) was used for its production. The cement–asbestos slate had been removed from a farm building in Upper Silesia, from around the city of Mikołów. It had been exposed to outside weather conditions for about 30 years.
Because the use of crocidolite asbestos was prohibited about 30 years ago, it was difficult to procure, not only “pure” crocidolite asbestos (as raw material), but also any products that contain it. Due to the very small amount of the obtained asbestos specimens and difficulties in obtaining their significant amount, they were generally characterized as received without any preliminary preparation. All of the asbestos samples were studied by differential thermal analysis (DTA) and thermogravimetry (TG/DTG). The mineralogical composition of both the natural and heated samples (after DTA study) was evaluated by X-ray diffraction (XRD).
To accurately determine the temperature range of the crocidolite dehydroxylation process, thermogravimetric analysis with evolved gas analysis (TG-EGA) for K1 and K2 sample was applied. Moreover the small amount of K2 sample was additional washed by hydrochloric acid (1:3 aqueous solution) to remove the grains of the cementitious matrix from the asbestos fibres, and then the TG-EGA measurement was determined. Based on the previous results, the isothermal calcination for 2 h at different selected temperatures was applied. The crocidolite asbestos samples were isothermally annealed at predetermined temperatures of 350, 400 and 450 °C as well as around 700 °C, i.e. the minimal temperature that was required in the case of the chrysotile asbestos thermal utilization [10, 12]. After thermal treatment in the laboratory furnace, the obtained fibre-like samples were subjected to a macroscopic examination and the study using the FT-IR method to follow the mineralogical change in the asbestos fibres. For a selected sample, scanning electron microscope observation (SEM) was also applied. Chemical analysis of used crocidolite asbestos sample was determined by the scanning electron microscopy in combination with energy dispersive X-ray analysis (SEM–EDX).
Thermal analysis (DTA and TG) was performed using a Paulik–Paulik–Erdey (MOM, Hungary) type derivatograph within the range of temperature of 20–1,000 °C. The conditions were the following: mass of the sample 200 mg, air atmosphere, heating rate 10 K min−1, platinum crucible and Al2O3 as the reference material.
Thermogravimetric analysis (TG) connected with evolved gas analysis (EGA) was both performed using a thermal analyser STA 409 PC NETZSCH that was coupled with a quadrupole mass spectrometer QMS 403 C Aëolos. A total of 60 mg of samples was placed in an alumina crucible. The heating rate of the sample was 5 K min−1. Testing was performed in air with a flow rate of 10 mL min−1.
XRD analysis of the examined samples was carried out using a Seifert XRD-3003 TT diffractometer (CuKα radiation, Ni filter, 40 kV, 30 mA).
IR spectra were measured on a Nicolett 6700 FT-IR spectrophotometer (ATR method).
The microstructure of the samples was examined by a scanning electron microscope (Hitachi TM-3000). Observations were made after coating the sample surfaces with a thin layer of gold in order to obtain conductivity. SEM-EDX analysis was obtained using electron microscope Phenom Prox (accelerating voltage of 15 kV) with fully integrated EDX solution.
Results and discussion
The results of DTA combined with TG/DTG measurement are reported in Fig. 1. The DTA curve for the K1 sample (crocidolite asbestos from the deposit in Republic of South Africa) shows one strong endothermic effect. The total change in mass is approximately 3 mass% and is achieved through a generally continuous mass change during the measurement. For the K2 sample (crocidolite asbestos separated from the asbestos–cement slate), the total mass change is much higher (~17 mass%). This phenomenon can be explained by the preparation method of sample. The crocidolite fibres were obtained by manual separation from the matrix and consequently the bundles of asbestos fibres contained some grains of cement. Significant mass loss obtained after thermal analysis study is connected with the thermal decomposition of compounds from cementitious matrix.
Around 400 and 650–700 °C, very weak endothermic effects are observed on the DTA curve of both tested samples. These effects are accompanied by slight mass changes that are more visible on the DTG curve. The first probably came from the dehydroxylation process of crocidolite asbestos, and the second may have been related to the thermal decomposition of some impurities, such as carbonates. A characteristic strong endothermic peak at T max 900–920 °C is common to both tested samples and is the sole one that is clearly visible for crocidolite samples. For the sample separated from the asbestos–cement slate (K2), a strong endothermic effect is also marked at approximately 800 °C. This is typical of the thermal decomposition of calcite, the grains of which remain on asbestos fibres after mechanical separation from the cementitious matrix. This effect is connected with a significant mass loss. For both samples in a temperature range of 600–700 °C, weak effects are visible, which may indicate the presence of other carbonate minerals (such as magnesite MgCO3). Furthermore, for K2 sample, this effect may suggest the thermal decomposition of a jennite-like (9CaO·6SiO2·11H2O) compound from the cementitious matrix [36–38] or decomposition of poorly crystallized calcite [39]. When the calcite is strongly weathered, the normal single-step decomposition reaction is gradually turn into a double-stage reaction with the clearly shift to lower temperature. The decomposition temperature of calcite with lower crystallinity is lower by about ~50 °C than that of the well-crystallized variation. This condition was fulfilled in relation to used asbestos–cement slate (the source of K2 sample), which about 30 years was on the building.
In both of the tested crocidolite samples, the endothermic peak at about 900 °C is without a mass loss and may indicate a physical transformation of the asbestos fibres that are melted. The obtained materials were brittle. SEM images of crocidolite asbestos after thermal treatment (after DTA/TG measurement) clearly show the presence of partially melted particles that were easy to crush (Fig. 2). This is in conformity with the results obtained by [31], where the authors observed no mass loss of crocidolite above ~700 °C and SEM observations showed a fibrous-like structure that was melt-bonded.
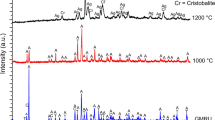
As a result of heat treatment up to 1,000 °C, material obtained from the crocidolite asbestos fibres (marked with *) exhibits a large X-ray amorphous nature (Fig. 3) and the XRD peaks show low intensity. This is clearly visible for the K2 sample. This phenomenon can be explained by the presence of additional Ca-containing compounds (e.g. calcite CaCO3) from cementitious matrix on the crocidolite fibres. In ceramic industry, Ca-containing compounds are considered as flux agent that promotes the sintering process and the formation of liquid phase. In the case of the K1 sample, a few peaks may indicate the presence of hematite and cristobalite as one of the products of thermal decomposition of crocidolite asbestos [30]. According to the literature [11, 34], one of the minerals that is created during thermal decomposition of crocidolite may be acmite (NaFeSi2O6). This pure mineral has an incongruent melting point with the separation of hematite at 990 ± 5 °C [40]. The presence of the molten phase after thermal treatment at 1,000 °C may indirectly indicate the earlier presence of acmite in the system and thereby the thermal decomposition of crocidolite asbestos samples.
Due to the absence of a clear result during DTA study, which would indicate the occurrence of the dehydroxylation process of crocidolite asbestos, additional TG-EGA studies were undertaken (Fig. 4). For the K1 sample (Fig. 4a), in a temperature range up to 550 °C, we observe a continuous mass loss with the total mass change of ~2.2 mass%. In this temperature range, a loss of adsorbed water can be identified from the surface of the asbestos fibres (the peak is at 110 °C on the DTG curve and at 149 °C on the EGA plot for H2O analysis). The weak peaks in the range of 250–550 °C on the DTG and EGA curves correspond to the combustion of organic matter (peaks at 360 °C and 507 °C for EGA of CO2). The most interesting is the effect of water release in the temperature range of 400–500 °C with a maximum at 463 °C on the H2O plot. This effect combined with mass loss is probably caused by the dehydroxylation process of crocidolite fibres. Based on the result, it can be stated that the dehydroxylation process takes place in K1 crocidolite asbestos sample in the assumed temperature range, i.e. 400–500 °C. In this temperature range, a thermal decomposition reaction of asbestos takes place, which can be generally written for an ideal chemical formula of crocidolite asbestos as:
In fact, the reaction is more complicated and is associated with oxidation. At this stage, the formation of oxycrocidolite Na2Fe4FeSi8O24 occurs [34]. The small loss of mass in the temperature range of 600–700 °C (with a maximum peak on the CO2 plot at 664 °C) comes from carbonate impurities, probably magnesite or calcite, which may be associated with asbestos deposits. A small increase in mass on the TG is observed in the temperature range of 900–950 °C (with a maximum peak on the DTG at ~925 °C). This phenomenon may come from the oxidation of remaining Fe2+ to Fe3+. According to [34], a small amount of Fe2+ (about 0.4 mass%) remains after heating crocidolite asbestos to constant mass in air at 950–1,000 °C. This amount is probably controlled by equilibrium and by not kinetic considerations. Since the oxycrocidolite is present, the oxidation occurs by mass gain in the second stage at higher temperature. According to Hodgson et al. [34], the second stage of oxidation occurs at 600–950 °C and it is difficult to observe because the decomposition of oxycrocidolite takes place:
and afterwards the spinel is being largely oxidized to hematite.
The TG-EGA plot for K2 sample (blue asbestos fibres separated from asbestos–cement sample) is shown in Fig. 4b. Mainly, the effects that come from cementitious matrix are visible in the plot. In the temperature range of 75–150 °C, mass loss (approximately 3 mass%) is visible in the thermogravimetric curves and it is accompanied by a peak in the H2O plot. This is adsorbed water and water from the thermal decomposition of the hydrates CSH phase [37]. Next, above the temperature 200 °C, the continuous mass loss is visible, which comes from the combustion of organic material traces. These traces can be formed as a result of long-term contact of asbestos–cement slate with the external environment. A small peak coming from H2O at 430 °C indicates the presence of portlandite (Ca(OH)2). This is one of the main minerals in the cement paste. At a higher temperature (in the range of 650–750 °C), the decomposition of carbonates takes place. This is indicated by a clearly visible loss of mass and a peak of CO2 at 707 °C in the flue gas.
The most important is the effect from H2O at 616 °C on the EGA curve (peak on the DTG at ~610 °C). This effect is associated with the thermal decomposition of crocidolite asbestos contained in the used asbestos–cement slate. It is more pronounced when the K2 sample was previously treated by hydrochloric acid (Fig. 4c), and carbonates compounds from cementitious matrix were decomposed.
Based on the theoretical chemical formula of crocidolite, the mass loss that is associated with the dehydroxylation process should be around 2.0 mass%. In this study, the mass change is 2.5 mass% (Fig. 4c). This value is close to the theoretical value and clearly suggests that the dehydroxylation process takes place in this crocidolite asbestos sample in the temperature range 550–700 °C.
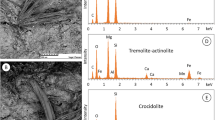
The differences in the decomposition noted between the samples K1, K2 and treated K2 could possibly be caused by the different chemical composition. In comparison with the K1 sample, the dehydroxylation temperature for the K2 samples is higher. This phenomenon can be explained as follows: according to Freeman [33], the temperature at which dehydroxylation occurs in amphiboles is largely dependent on the type of cation that occupies the M 3 and M 1 sites in the crystal structure, because in the amphibole structure the hydroxyl group is bonded to the cations that occupy these sites. The temperature of dehydroxylation is shown to rise with increasing Mg2+ content in the sites. In amphibole minerals, these sites are occupied in various proportions by Mg2+ or Fe2+. This is also true in crocidolite asbestos where a part of iron ions may be replaced by magnesium ions. The content of Mg calculated as MgO in crocidolite asbestos sample from different mineral deposits varies within a wide range. Table 1 shows the results of chemical analysis (available from the literature [2, 29, 41] ) of crocidolite asbestos, which have been taken from the various deposits in the world. In general, the content of the main components in all deposits is similar. There are only clearly visible differences in the content of FeO and MgO. According to literature, the exemplary deposits of crocidolite from the Republic of South Africa contained several mass percentage of MgO, while for soviet deposits this value achieves even 10.4 mass%. According to Freeman [33], this difference shifts the dehydroxylation temperature to higher temperature. The SEM–EDX analysis confirmed this phenomenon and the obtained results show that for K2 sample the content of Mg is clearly higher than for the K1 sample. Selected SEM images of crocidolite asbestos samples in combination with a point chemical analysis are presented in Fig. 5.
The results of isothermal calcinations of crocidolite asbestos samples confirm the fact that crocidolite asbestos undergoes thermal decomposition at a temperature below 700 °C. With the increase in the heat treatment temperature, the characteristic bluish colour of the crocidolite fibres shifted into rust-red (Fig. 6). This was due to the oxidation process of iron from Fe2+ to Fe3+. As a result, the fibres also became more fragile and brittle. Significant fragmentation was observed even with light hand grinding (Fig. 6e). Thermal decomposition of crocidolite asbestos and the process of its dehydroxylation were confirmed by infrared studies (Fig. 7). The characteristic double spectroscopic band that comes from stretch vibration of the OH groups at ~3,600 cm−1 disappears with the increase in the thermal treatment temperature. This band may be the clear evidence of crocidolite presence in the sample [42]. After calcination at 450 °C for 2 h, it is not visible for all of the testes samples. The band’s absence in the high-range wave numbers at 3,640–3,680 cm−1, which correspond to OH stretching, admittedly confirms thermal decomposition of this amphibole-type asbestos. The IR bands recorded at ~1,400, 870 and 775 cm−1 for the K2 sample are typical of carbonate compounds (such as CaCO3 from the cementitious matrix and/or MgCO3). With an increase in thermal treatment temperature above 600 °C, its intensity clearly decreases. This indicates the initiation of thermal decomposition of calcite. For the K2 sample treated by acid, this band is absent. The IR bands recorded in the region of 1,080–935 cm−1 for the considered sample are typical of the Si–O–Si stretches in the silica network. The final confirmation of the crocidolite asbestos decomposition was accomplished by the SEM observations (Fig. 8). Although the fibrous form is retained after being removed from the furnace, gentle grinding causes significant generation of powder form that is free from hazardous properties.
Conclusions
Thermal treatment is one of the possible methods of asbestos detoxification. As a result of this process, the structure of crocidolite asbestos is changed. The study showed that the dehydroxylation process of crocidolite asbestos is dependent on the origin of the sample and takes place in the range of 400–700 °C. This temperature range, in spite of all, is much smaller than that reported in the literature. After isothermal annealing of crocidolite asbestos, the minimal temperature of thermal treatment decreases and the dehydroxylation process for all of the tested samples occurs in the temperature range 400–450 °C. The obtained material is brittle, has high grinding ability and is easily milled to pulverulent-shape material. The obtained product of thermal treatment after soft crush does not contain respirable fibres, which are the cause of various illnesses. This product can be used as a safe material for further application.
References
Sporn TA. Mineralogy of asbestos. In: Tannapfel A, editor. Malignant mesothelioma. Berlin: Springer; 2011. p. 1–11.
Virta RL. Mineral commodity profiles—asbestos. Reston: U.S. Geological Survey Circular; 2005.
Harris LV, Kahwa IA. Asbestos: old foe in 21st century developing countries. Sci Total Environ. 2003;307:1–9.
Pyssa J, Rokita GM. Azbest–występowanie, wykorzystanie i sposób postępowania z odpadami azbestowymi. Gospod Surow Mineral. 2007;31:49–61 (in Polish).
LaDou J, Castleman B, Frank A, Gochfeld M, Greenberg M, Huff J, Joshi TK, Landrigan PJ, Lemen R, Myers J, Soffritti M, Soskolne CL, Takahashi K, Teitelbaum D, Terracini B, Watterson A. The case for global ban on asbestos. Environ Health Perspect. 2010;118:897–901.
Demirogiu H. Hazard of white asbestos. Lancet. 1998;352:322–3.
Quinlan TR, Berube KA, Hacker MP, Taatjes DJ, Timblin CR, Goldberg J, Kimberley P, O’Shaughnessy P, Hemenway D, Torino J, Jimenez LA, Mossman BT. Mechanisms of asbestos-induced nitric oxide production by rat alveolar macrophages in inhalation and in vitro models. Free Radic Biol Med. 1998;24:778–88.
Więcek E. Azbest—narażenie i skutki zdrowotne. Bezp Pracy. 2004;2:2–6.
Dyczek J. Eksploatacja i usuwanie wyrobów zawierających azbest. Mater Bud. 2006;11:46–8 (in Polish).
Kusiorowski R, Zaremba T, Piotrowski J, Adamek J. Thermal decomposition of different types of asbestos. J Therm Anal Calorim. 2012;109:693–704.
Jeyaratnam M, West NG. A study of heat-degraded chrysotile, amosite and crocidolite by X-ray diffraction. Ann Occup Hyg. 1994;38:137–48.
Zaremba T, Krząkała A, Piotrowski J, Garczorz D. Study on the thermal decomposition of chrysotile asbestos. J Therm Anal Calorim. 2010;101:479–85.
Dellisanti F, Minguzzi V, Morandi N. Experimental results from thermal treatment of asbestos containing materials. GeoActa 2001–2002;1:61–70.
MacKenzie KJD, Meinhold RH. Thermal reactions of chrysotile revisited: a 29Si and 25Mg MAS NMR study. Am Mineral. 1994;79:43–50.
Datta AK, Samantaray BK, Bhattacherjee S. Thermal transformation in a chrysotile asbestos. Bull Mater Sci. 1986;8:497–503.
Jolicoeur C, Duchesne D. Infrared and thermogravimetric studies of the thermal degradation of chrysotile asbestos fibers: evidence for matrix effects. Can J Chem. 1981;59:1521–6.
Martin CJ. The thermal decomposition of chrysotile. Mineral Mag. 1977;41:453–9.
Teixeira APC, Santos EM, Vieira AFP, Lago RM. Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synthesis. Chem Eng J. 2013;232:104–10.
Datta AK. Dehydration of chrysotile asbestos: an infrared absorption study. J Mater Sci Lett. 1991;10:870–1.
Zaremba T, Peszko M. Investigation of the thermal modification of asbestos wastes for potential use in ceramic formulation. J Therm Anal Calorim. 2008;92:873–7.
Cattaneo A, Gualtieri AF, Artioli G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys Chem Minerals. 2003;30:177–83.
Boccaccini DN, Leonelli C, Rivasi MR, Romagnoli M, Veronesi P, Pellacani GC, Boccaccini AR. Recycling of microwave inertised asbestos containing waste in refractory materials. J Eur Ceram Soc. 2007;27:1855–8.
Malkov AA, Korytkova EN, Maslennikova TP, Shtykhova AM, Gusarov VV. Effect of heat treatment on structural-chemical transformations in magnesium hydrosilicate [Mg3Si2O5(OH)4] nanotubes. Russ J Appl Chem. 2009;82:2079–86.
Zulumyan N, Mirgorodski A, Isahakyan A, Beglaryan H. The mechanism of decomposition of serpentines from peridotites on heating. J Therm Anal Calorim. 2014;115:1003–12.
Gualtieri AF, Tartaglia A. Thermal decomposition of asbestos and recycling in traditional ceramics. J Eur Ceram Soc. 2000;20:1409–18.
Virta RL. Asbestos: geology, mineralogy, mining and uses. Reston: U.S. Geological Survey Open-file report 02-149; 2002.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens. Part C: Arsenic, metals, fibres, and dusts. Lyon; 2009.
Łuniewski A, Łuniewski S. Azbest. Historyczne obciążenie z XX wieku. Białystok: Wydawnictwo Ekonomia i Środowisko; 2009 (in Polish).
Le Cilliers JJ, Freeman AG, Hodgson AA, Taylor HFW. Crocidolite from the Koegas-Westerberg area, South Africa. Econ Geol. 1961;56:1421–37.
Hodgson AA. The thermal decomposition of miscellaneous crocidolites. Mineral Mag. 1965;35:291–305.
Fujishige M, Kuribara A, Karasawa I, Kojima A. Low-temperature pyrolysis of crocidolite and amosite using calcium salts as a flux. J Ceram Soc Jpn. 2007;115:434–9.
Gualtieri AF, Levy D, Belluso E, Dapiaggi M. Kinetics of the decomposition of crocidolite asbestos: a preliminary real-time X-ray powder diffraction study. Mater Sci Forum. 2004;443–444:291–4.
Freeman AG. The dehydroxylation behaviour of amphiboles. Mineral Mag. 1966;35:953–7.
Hodgson AA, Freeman AG, Taylor HFW. The thermal decomposition of crocidolite from Koegas, South Africa. Mineral Mag. 1965;35:5–30.
Addison CC, Addison WE, Neal GH, Sharp JH. Amphiboles. Part I. The oxidation of crocidolite. J Chem Soc. 1962;278:1468–71.
Dias CMR, Cincotto MA, Savastano H, John VM. Long-term aging of fiber-cement corrugated sheets—the effect of carbonation, leaching and acid rain. Cem Concr Compos. 2008;30:255–65.
Stepkowska ET, Blanes JM, Real C, Perez-Rodriguez JL. Hydration products in two aged cement pastes. Thermochim Acta. 2004;420:79–87.
Kusiorowski R, Zaremba T, Piotrowski J, Gerle A. Thermal decomposition of asbestos-containing materials. J Therm Anal Calorim. 2013;113:179–88.
Földavári M. Handbook of thermogravimetric system of minerals and its use in geological practice. Budapest: Geological Institute of Hungary; 2011.
Yagi K. The system acmite-diopside and its bearing on the stability relations of natural pyroxenes of the acmite-hedenbergite-diopside series. Am Mineral. 1966;51:976–1000.
Łącki JW. Wydobycie i zastosowanie azbestu. Katowice: Śląsk; 1974 (in Polish).
Marconi A. Application of infrared spectroscopy in asbestos mineral analysis. Ann Ist Sup Sanita. 1983;19:629–38.
Acknowledgements
The authors want to thank Mr. P. Wyszomirski and W. Pichór for the help in obtaining sample of pure crocidolite asbestos. We are also indebted to the anonymous referees for their comments that greatly improved the quality of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kusiorowski, R., Zaremba, T., Gerle, A. et al. Study on the thermal decomposition of crocidolite asbestos. J Therm Anal Calorim 120, 1585–1595 (2015). https://doi.org/10.1007/s10973-015-4421-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4421-7