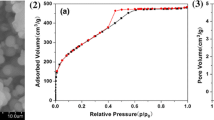
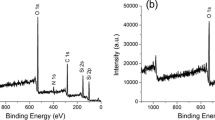
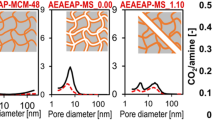
Abstract
Due to the different rates of silica alkoxide hydrolysis and furfuryl alcohol polymerization, hydrophobic poly(furfuryl alcohol) network became incompatible with hydrophilic silica network, PFA-rich spheres were formed in the sol–gel process. The presence of the amphiphilic triblock copolymers P123 could help reduce the degree of phase separation. Slower drying rate, higher aging temperature, and higher C/SiO2 ratio accelerated the polymerization of FA and tend to aggregate more PFA spherical domains. However, in the sample with a fast increased viscosity, the temperature effect would be small since the PFA is hard to agglomerate, leading a homogenous mesoporous structure. If the C/SiO2 ratio is quite high to get a more hydrophobic system, there will be no phase separation too. The understanding of the phase separation of poly-furfuryl alcohol-silica systems offers great opportunities in controlling of the mesoporous carbon-silica nanocomposites.
Graphical Abstract
Schematic diagrams of the formation of PFA/silica hybrids: during the sol–gel process, once an alkoxide molecule is hydrolyzed, the resulting hydroxy groups make the molecule more polar, which are more hydrophilic. At the same time, FA polymerized to form PFA oligomers, which are hydrophobic. The incompatibility between these two precursors could be reduced by the amphiphilic triblock copolymers P123, because the long PEO chains are hydrophilic while the PPO block is hydrophobic. Either homogenous or phase separated PFA/silica/P123 can be obtained by tailoring the chemical ratio and the sol–gel process parameters. 






Similar content being viewed by others
References
Yao H, Gao M, Yu S (2010) Small organic molecule templating synthesis of organic-inorganic hybrid materials: their nanostructures and properties. Nanoscale 2:323–334
Karatas S, Hosgor Z, Apohan N, Gungor A (2010) Preparation and characterization of photopolymerizable organic-inorganic hybrid materials by the sol-gel method. J Polym Res 17:247–254
Spange S, Grund S (2009) Nanostructured organic-inorganic composite materials by twin polymerization of hybrid monomers. Adv Mater 21:2111–2116
Luo Y (2008) Coordination-induced formation of nanobelts of organic-inorganic hybrid materials at room temperature. Mater Lett 62:3549–3551
Ma M, Sun Z, Zhu Y, Zhang G, Sun T, Li W, Luo H (2014) Two novel oxovanadium-organophosphonate hybrids with a 3D supramolecular structure: synthesis, crystal structures, surface photovoltage and luminescent properties. RSC Adv 4:46595–46601
Duering J, Hoelzer A, Kolb U, Branscheid R, Groehn F (2013) Supramolecular organic-inorganic hybrid assemblies with tunable particle size: Interplay of three noncovalent interactions. Angew Chem Int Ed 52:8742–8745
Pal N, Bhaumik A (2013) Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv Colloid Interface Sci 189:21–41
Fuentes-Alventosa JM, Introzzi L, Santo N, Cerri G, Brundu A, Farris S (2013) Self-assembled nanostructured biohybrid coatings by an integrated ‘sol-gel/intercalation’ approach. RSC Adv 3:25086–25096
Suriyanon N, Punyapalakul P, Ngamcharussrivichai C (2015) Synthesis of periodic mesoporous organosilicas functionalized with different amine-organoalkoxysilanes via direct co-condensation. Mater Chem Phys 149:701–712
Falcao AN, Carrapico M, Sousa JS, Margaca F, Ferreira LM, Carvalho FG, Salvado I, Teixeira J (2003) Investigation of organic-inorganic hybrid materials prepared by irradiation. J Sol-Gel Sci Techn 26:349–352
Wang D, Liu W, Feng Q, Dong C, Liu Q, Duan L, Huang J, Zhu W, Li Z, Xiong J, Liang Y, Chen J, Sun R, Bian L, Wang D (2017) Effect of inorganic/organic ratio and chemical coupling on the performance of porous silica/chitosan hybrid scaffolds. Mat Sci Eng C-Mater 70:969–975
Rashti A, Yahyaei H, Firoozi S, Ramezani S, Rahiminejad A, Karimi R, Farzaneh K, Mohseni M, Ghanbari H (2016) Development of novel biocompatible hybrid nanocomposites based on polyurethane-silica prepared by sol gel process. Mat Sci Eng C-Mater 69:1248–1255
Petcu C, Purcar V, Ianchis R, Spataru C, Ghiurea M, Nicolae CA, Stroescu H, Atanase L, Frone AN, Trica B, Donescu D (2016) Synthesis and characterization of polymer-silica hybrid latexes and sol-gel-derived films. Appl Surf Sci 389:666–672
Aleshin AN, Krylov PS, Berestennikov AS, Shcherbakov IP, Petrov VN, Kondratiev VV, Eliseeva SN (2016) The redox nature of the resistive switching in nanocomposite thin films based on graphene (graphene oxide) nanoparticles and poly (9-vinylcarbazole). Synth Met 217:7–13
Gao D, Liang Z, Lyu B, Ma J (2016) Organic/Inorganic nanocomposites prepared by miniemulsion polymerization. Prog Chem 28:1076–1083
Kumar KS, Sanyadanam S, Paik P (2016) Dangling ultrafine nano silica on graphene oxide to form hybrid nanocomposite: enhancement of dielectric properties. Mater Res Express 3:055019
Besnard R, Arrachart G, Cambedouzou J, Pellet-Rostaing S (2016) Tuning the nanostructure of highly functionalized silica using amphiphilic organosilanes: curvature agent effects. Langmuir 32:4624–4634
Catauro M, Mozzati MC, Bollino F (2015) Sol-gel hybrid materials for aerospace applications: chemical characterization and comparative investigation of the magnetic properties. Acta Astronaut 117:153–162
Basturk E, Oktay B, Kahraman MV (2015) Dual-crosslinked thiol-ene/sol gel hybrid electrospun nanowires: preparation and characterization. J Polym Res 22:133
Kiasat AR, Nazari S, Davarpanah J (2014) Facile synthesis of an organic-inorganic nanocomposite, PEG-silica, by sol-gel method; its characterization and application as an efficient catalyst in regioselective nucleophilic ring opening of epoxides: preparation of beta-azido alcohols and beta-cyanohydrins. C R Chim 17:124–130
Tsuru K, Shirosaki Y, Hayakawa S, Osaka A (2013) Sol-Gel-derived silicate nano-hybrids for biomedical applications. Biol Pharm Bull 36:1683–1687
Turrin CO, Maraval V, Caminade AM, Majoral JP, Mehdi A, Reye C (2000) Organic-inorganic hybrid materials incorporating phosphorus-containing dendrimers. Chem Mater 12:3848–3856
de Ferri L, Lorenzi A, Lottici PP (2016) OctTES/TEOS system for hybrid coatings: real-time monitoring of the hydrolysis and condensation by Raman spectroscopy. J Raman Spectrosc 47:699–705
Haryadi H, Sugianto D, Ristopan E (2015) Development of Composite Membranes of PVA-TEOS doped KOH for Alkaline Membrane Fuel Cell. In: Hadiyanto, Nur H, Budiman A, Iskandar F, Ismadji S (eds) AIP Conference Proceedings
Almeida JC, Castro AGB, Lancastre JJH, Miranda Salvado IM, Margaca FMA, Fernandes MHV, Ferreira LM, Casimiro MH (2014) Structural characterization of PDMS-TEOS-CaO-TiO2 hybrid materials obtained by sol-gel. Mater Chem Phys 143:557–563
Khovanets G, Medvedevskikh Y, Sezonenko T, Musiy R, Zakordonskiy V (2014) The Polymer-Silica Nanocomposites of the System HEMA - TEOS. 2014 IEEE International Conference on Oxide Materials for Electronic Engineering: 98-99
Shilova OA (2013) Synthesis and structure features of composite silicate and hybrid TEOS-derived thin films doped by inorganic and organic additives. J Sol-Gel Sci Techn 68:387–410
Kim S, Lee P, Bano S, Park Y, Nam S, Lee K (2016) Effective incorporation of TiO2 nanoparticles into polyamide thin-film composite membranes. J Appl Polym Sci 133
Kassner L, Nagel K, Gruetzner RE, Korb M, Rueffer T, Lang H, Spange S (2015) Polyamide 6/silica hybrid materials by a coupled polymerization reaction. Polym Chem 6:6297–6304
Moghanian H, Mobinikhaledi A, Monjezi R (2015) Synthesis and characterization of novel aliphatic-aromatic polyamide/Fe3O4 nanocomposites containing pendent 9H-xanthene groups. Des Monomers Polym 18:157–169
Kanamori K (2013) Liquid-phase synthesis and application of monolithic porous materials based on organic-inorganic hybrid methylsiloxanes, crosslinked polymers and carbons. J Sol-Gel Sci Techn 65:12–22
Wang F, Zhao L, Fang W, He X, Liang F, Chen H, Chen H, Du X (2016) Preparation of organic/inorganic composite phenolic resin and application in Al2O3-C refractories. Int J Appl Ceram Tec 13:133–139
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M (2016) Organic-inorganic nanohybrids of novolac phenolic resin and carbon nanotube: High carbon yields by using carbon nanotube aerogel and resin incorporation into aerogel network. Micropor Mesopor Mat 224:58–67
Paydayesh A, Kokabi M (2015) Highly filled organoclay/phenolic resin nanocomposite as an ablative heat shield material. Iran Polym J 24:389–397
Schuetz MR, Sattler K, Deeken S, Klein O, Adasch V, Liebscher CH, Glatzel U, Senker J, Breu J (2010) Improvement of thermal and mechanical properties of a phenolic resin nanocomposite by in situ formation of silsesquioxanes from a molecular precursor. J Appl Polym Sci 117:2272–2277
Yao JF, Wang HT, Chan KY, Zhang LX, Xu NP (2005) Incorporating organic polymer into silica walls: A novel strategy for synthesis of templated mesoporous silica with tunable pore structure. Micropor Mesopor Mat 82:183–189
Yao J, Wang H, Zhang X, Zhu W, Wei J, Cheng Y (2007) Role of pores in the carbothermal reduction of carbon-silica nanocomposites into silicon carbide nanostructures. J Phys Chem C 111:636–641
Goegelein C, Naegele G, Buitenhuis J, Tuinier R, Dhont JKG (2009) Polymer depletion-driven cluster aggregation and initial phase separation in charged nanosized colloids. J Chem Phys 130:204905
Miyamoto M, Nagata K, Maruo T, Nishiyama N, Yogo K, Egashira Y, Ueyama K (2008) Highly permeable mesoporous silica membranes synthesized by vapor infiltration of tetraethoxysilane into non-ionic alkyl poly(oxyethylene) surfactant films. J Membr Sci 325:698–703
Song L, Wu Q, Vogt BD (2008) Templating multiple length scales by combining phase separation, self-assembly and photopatterning in porous films. J Colloid Interf Sci 328:374–384
Nakanishi K, Sagawa Y, Soga N (1991) Pore surface characteristics of macroporous silica gels prepared from polymer-containing solution. J Non-Cryst Solids 134:39–46
Nakanishi K, Soga N (1992) Phase separation in silica sol-gel system containing polyacrylic acid. III. Effect of catalytic condition. J Non-Cryst Solids 142:36–44
Nakanishi K, Soga N (1992) Phase separation in silica sol-gel system containing polyacrylic acid II. Effects of molecular weight and temperature. J Non-Cryst Solids 139:14–24
Nakanishi K, Soga N (1992) Phase separation in silica sol-gel system containing polyacrylic acid I. Gel formaation behavior and effect of solvent composition. J Non-Cryst Solids 139:1–13
Nakanishi K, Soga N (1992) Phase separation in silica sol-gel system containing polyacrylic acid. IV. Effect of chemical additives. J Non-Cryst Solids 142:45–54
Nakanishi K, Nagakane T, Soga N (1998) Designing double pore structure in Alkoxy-derived silica incorporated with nonionic surfactant. J Popous Mat 5:103–110
Grund S, Seifert A, Baumann G, Baumann W, Marx G, Kehr M, Spange S (2006) Monolithic silica with bimodal pore size distribution fabricated by self-separated sol-gel composite materials. Micropor Mesopor Mat 95:206–212
Sun YW, Wang YJ, Guo W, Wang T, Luo GS (2006) Triblock copolymer and poly(ethylene glycol) as templates for monolithic silica material with bimodal pore structure. Micropor Mesopor Mat 88:31–37
Takahashi R, Sato S, Sodesawa T, Suzuki K, Tafu M, Nakanishi K, Soga N (2001) Phase separation in sol-gel process of alkoxide-derived silica-zirconia in the presence of polyethylene oxide. J Am Ceram Soc 84:1968–1976
Zhang H, Hardy GC, Khimyak YZ, Rosseinsky MJ, Cooper AI (2004) Synthesis of hierarchically porous silica and metal oxide beads using emulsion-templated polymer scaffolds. Chem Mater 16:4245–4256
Nakanishi K (2007) Functional porous materials via sol-gel with phase separation. J Ceram Soc Jpn 115:169–175
Konishi J, Fujita K, Oiwa S, Nakanishi K, Hirao K (2008) Crystalline ZrO2 monoliths with well-defined macropores and mesostructured skeletons prepared by combining the alkoxy-derived sol-gel process accompanied by phase separation and the solvothermal process. Chem Mater 20:2165–2173
Wang K, Yao J, Wang H, Cheng Y (2008) Effect of seeding on formation of silicon carbide nanostructures from mesoporous silica-carbon nanocomposites. Nanotechnology 19:175605
Wang K, Wang H, Cheng Y (2010) Synthesis of nanostructured silicon carbide spheres from mesoporous C-SiO2 nanocomposites. Chem Commun 46:303–305
Manocha SM, Vashistha DY, Manocha LM (1997) Studies of the pyrolysis behaviour of silica sol copolymerized with furfuryl alcohol. J Mater Sci Lett 16:705–707
Spange S, Muller H, Jager C, Bellmann C (2002) Fabrication of carbon/silica hybrid materials using cationic polymerization and the sol-gel process. Macromol Symp 177:111–124
Zarbin A, Bertholdo R, Oliveira M (2002) Preparation, characterization and pyrolysis of poly(furfuryl alcohol)/porous silica glass nanocomposites: novel route to carbon template. Carbon 40:2413–2422
Wang H, Yao J (2006) Use of poly(furfuryl alcohol) in the fabrication of nanostructured carbons and nanocomposites. Ind Eng Chem Res 45:6393–6404
Yao J, Wang H, Zhang X, Zhu W, Wei J, Cheng Y (2007) Role of pores in the carbothermal reduction of carbon-silica nanocomposites into silicon carbide nanostructures. J Phys Chem C 111:636–641
Wan Y, Shi Y, Zhao D (2008) Supramolecular aggregates as templates: ordered mesoporous polymers and carbons. Chem Mater 20:932–945
Flory PJ (1942) Thermodynamics of high polymer solutions. J Chem Phys 10:51
Huggins ML (1942) Thermodynamic properties of solutions of long-chain compounds. Ann NY Acad Sci 43:1–32
K. SWS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619
Ben-Arfa BAE, Miranda Salvado IM, Ferreira JMF, Pullar RC (2017) Novel route for rapid sol-gel synthesis of hydroxyapatite, avoiding ageing and using fast drying with a 50-fold to 200-fold reduction in process time. Mat Sci Eng C-Mater 70:796–804
Sakuragi A, Igarashi Y, Kajihara K, Kanamura K (2016) Synthesis of silanol-rich long-life polysilsesquioxane liquids by cosolvent-free hydrolytic polycondensation of organotrimethoxysilanes followed by aging. Dalton Trans 45:3151–3157
Soderzhinova MM, Tarasova DV, Chibirova FK (2016) Aging of titania hydrosols prepared via ultrasonic processing. Inorg Mater 2:470–475
Veliscek-Carolan J, Knott R, Hanley T (2015) Effects of precursor solution aging and other parameters on synthesis of ordered mesoporous titania powders. J Phys Chem C 119:7172–7183
Letaief N, Lucas-Girot A, Oudadesse H, Meleard P, Pott T, Jelassi J, Dorbez-Sridi R (2014) Effect of aging temperature on the structure, pore morphology and bioactivity of new sol-gel synthesized bioglass. J Non-Cryst Solids 402:194–199
Zhong H, Zhu G, Yang J, Wang P, Yang Q (2007) Periodic mesoporous hybrid monolith with hierarchical macro-mesopores. Micropor Mesopor Mat 100:259–267
Kim EJ (1995) Low pressure chemical vapor deposition of silicon dioxide films by thermal decomposition of tetra-alkoxysilanes. J Electrochem Soc 142:676
Kang KK, Rhee HK (2005) Synthesis and characterization of novel mesoporous silica with large wormhole-like pores: use of TBOS as silicon source. Micropor Mesopor Mat 84:34–40
Miyake Y, Yumoto T, Kitamura H, Sugimoto T (2002) Solubilization of organic compounds into as-synthesized spherical mesoporous silica. Phys Chem Chem Phys 4:2680–2684
Miyake Y, Kato T (2005) The formation process of spherical mesoporous silica with reverse nanostructure of MCM41 in a two-phase system. J Chem Eng Jpn 38:60–66
Miyake Y, Hanaeda M, Asada M (2007) Separation of organic compounds by spherical mesoporous silica prepared from W/O microemulsions of tetrabutoxysilane. Ind Eng Chem Res 46:8152–8157
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120:6024–6036
Kim SS, Pauly TR, Pinnavaia TJ (2000) Non-ionic surfactant assembly of ordered, very large pore molecular sieve silicas from water soluble silicates. Chem Commun 17:1661–1662
Kim SS, Pauly TR, Pinnavaia TJ (2000) Non-ionic surfactant assembly of wormhole silica molecular sieves from water soluble silicates. Chem Commun 10:835–836
Spange S, Schütz H, Martinez R (1993) [Composites from furfuryl alcohol and inorganic solids by cationic initiation, 2. Spectroscopic studies of poly(furfuryl alcohol)-silica composites obtained by trifluoroacetic acid initiation]. Die Makromol Chem 194:1537–1544
Spange S, Winkelmann H, Martinez R (1993) [Composites from furfuryl alcohol and inorganic solids by cationic initiation, 3. Scanning electron microscopic investigations of solid poly(furfuryl alcohol)-silica composites]. Die Angew Makromol Chem 208:125–131
Spange S, Heublein B, Schramm A, Martinez R (1992) [Composites from furfuryl alcohol and inorganic solids by cationic initiation, 1. General features]. Die Makromol Chem 13:511–515
Acknowledgements
K.W. thanks the financial support of this work from the “the Fundamental Research Funds for the Central Universities (WUT: 2017IVA088). This work is also supported by State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (Wuhan University of Technology). The authors acknowledge use of facilities within the Monash Center for Electron Microscopy for TEM and SEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, K., Wang, H. & Cheng, YB. Morphology control of mesoporous silica-carbon nanocomposites via phase separation of poly(furfuryl alcohol) and silica in the sol–gel synthesis. J Sol-Gel Sci Technol 82, 664–674 (2017). https://doi.org/10.1007/s10971-017-4358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4358-3