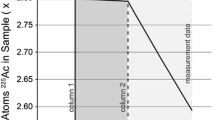
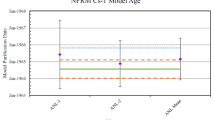
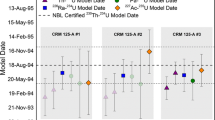
Abstract
Age-dating of 137Cs ceramic sources is shown to be a viable technology for nuclear forensics investigations. The 137Cs age-dating method in general, however, could be substantially improved by using radiometric rather than ICP-MS measurement of the 137Cs isotope and by refining the Cs/Ba separation process. With these improvements, uncertainty in the age of a 60-year-old source decreases from 1.35 to 0.68 y.





Similar content being viewed by others
References
Sommers J, Cummings D, Giglio J, Carney K (2009) Age determination of irradiated materials utilizing inductively coupled plasma mass spectrometric (ICP-MS) detection. J Radioanal Nucl Chem 282:591–595
Goldberg M, Chamberlain D, Kalensky M, Lewis M, Mertz C, Tsai Y (2009) Age-Dating and Analysis of ‘Ames’ 137Cs Sealed Source Material, Final Report. Argonne National Laboratory Technical Report ANL-NTNFC-09-0022
National Research Council Committee on Radiation Source Use and Replacement (2008) Radiation sources in the United States and their uses and origins: abbreviated version. National Research Council of the National Academies, National Academies Press, Washington, D.C
Steeb JL, Mertz CJ, Sandi G, Bass DA, Graczyk DG, Goldberg MM (2012) Micro-separations of cesium and barium in glass. J Radioanal Nucl Chem 292:757–762
Steeb JL, Graczyk DG, Tsai Y, Mertz CJ, Essling AM, Sullivan VS, Carney KP, Finck MR, Giglio JJ, Chamberlain DB (2013) Application of mass spectrometric isotope dilution methodology for 90Sr age-dating with measurements by thermal ionization and inductively coupled plasma mass spectrometry. J Anal At Spectrom 28:1493–1507
Ellison SLR, Rosslein M, Williams A (eds) (2000) Quantifying uncertainty in analytical measurements, Eurachem/CITAC Guide CG 4, 2nd edition QUAM:2000.1
Dejsupa C, Heo NH, Seff K (1989) Crystal structure of cesium zeolite A prepared by complete aqueous exchange. Zeolites 9:146–151
Schultz FJ, Tompkins JA, Haff KW, Case FN (1981) Preparation and characterization of cesium-137 aluminosilicate pellets for radioactive source applications. Oak Ridge National Laboratory technical report ORNL-5775
Vereshchagina TA, Vasilieva NG, Vereshchagin SN, Paretskov EN, Zykova ID, Krucheck DM, Manakova LF, Tretyakov AA, Anshits AG (2006) Porous materials based on cenospheres of coal fly ash for fixation of 137Cs and 90Sr in mineral-like aluminosilicates. Mater Res Soc Symp Proc Vol 932
Kaminski MD, Mertz CJ, Ferrandon M, Dietz NL, Sandi G (2009) Physical properties of an aluminosilicate waste form for cesium and strontium. J Nucl Mater 392:510–518
Enomoto S, Maeda S, Senoo M (1989) A simplified method for preparation of 137Cs pollucite γ-ray source. Int J Appl Radiat Isot 32:595–599
Milearski M (1989) Preparation of 137Cs pollucite source core. Isot Environ Health Stud 25:404–408
Scheetz BE, Aggarwal DK, Breval E, Roy R (1994) Sodium Zirconium Phosphate (NZP) as a host structure for nuclear waste immobilization: a review. Waste Manag 14:489–505
Chourasia RB, Ambastha RD, Shrivastava OP, Wattal PK (2010) Crystallographic evaluation of sodium zirconium phosphate as a host structure for immobilization of cesium. J Mater Sci 45:533–545
Richerson DW (2006) Modern ceramic engineering: properties, processing, and use in design. CRC Press, Boca Raton, FL
Schlesinger HI, Brown HC, Mayfield DL, Gilbreath JR (1953) Procedures for the preparation of methyl borate. J Am Chem Soc 75:213–215
Salit ML, Turk GC (1998) A Drift Correction Procedure. Anal Chem 70:3184–3190
Salit ML, Vocke RD, Kelly WR (2000) An ICP-OES method with 0.2 % expanded uncertainty for the characterization of LiAlO2. Anal Chem 72:3504–3511
Salit ML, Turk GC, Lindstrom AP, Butler TA, Beck CM, Norman B (2001) Single element solution comparisons with a high-performance inductively coupled plasma optical emission spectrometric method. Anal Chem 73:4821–4829
Berglund M, Wieser ME (2011) Isotopic compositions of the elements, 2009. Pure Appl Chem 83:397–410
Charbonneau L, Benoit JM, Jovanovic S, St-Amant N, Kiser S, Cooke MW, Mercier JF, Nielsen K, Kelly D, Samuleev P, Galea R, Moore K, Saul PRB, Chamberlain DB, Steeb JL, Graczyk DG, Tsai Y, Sullivan VS, Dimayuga IC, Shi Y, Rao R, Lariviere DA (2014) Nuclear forensic method for determining the age of radioactive cobalt sources. Anal Methods 6:983–992
Nelson F (1964) Ion exchange procedures: V. Separation of barium and radium. J. Chromatogr 16:403–406
Ditchburn RG, Whitehead NE (1995) Separation of Ra and Th from rock matrices for alpha-spectrometry. J Radioanal Nucl Chem 189:115–125
Gleason G (1980) In: Lyons W (ed) Radioelement analysis progress and problems. Ann Arbor Science, Ann Arbor
Reilly CN, Schmid RW, Sadek FS (1959) Chelon approach to analysis: I. Survey of theory and application. J Chem Ed 36:555–564
Evaluated Nuclear Structure Data File (ENDSF), September 2005
Acknowledgments
Argonne National Laboratory’s work was funded by the U.S. Department of Homeland Security. The submitted manuscript includes information created by UChicago Argonne, LLC, operator of Argonne National Laboratory (“Argonne”). Argonne, a U.S. Department of Energy Office of Science laboratory, is operated under Contract No. DE-AC02-06CH11357. The U.S. Government retains for itself, and others acting on its behalf, a paid-up nonexclusive, irrevocable worldwide license in said article to reproduce, prepare derivative works, distribute copies to the public, and display publicly, by or on behalf of the Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steeb, J.L., Graczyk, D.G., Tsai, Y. et al. Age-dating methodology for 137Cs ceramic sources. J Radioanal Nucl Chem 309, 999–1019 (2016). https://doi.org/10.1007/s10967-016-4712-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4712-x