Abstract
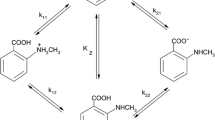
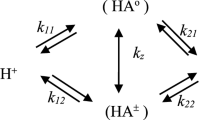
The solute–solvent interactions of alanine and valine were investigated potentiometrically in different aqueous solvent mixtures (0–50 % v/v) of methanol (an amphiprotic solvent) and tetrahydrofuran (a dipolar aprotic solvent). The macroscopic and microscopic protonation constants as well as the tautomeric constants were determined and correlated with either a macroscopic parameter such as dielectric constant or a microscopic parameter such as a Kamlet, Abboud, and Taft (KAT) solvatochromic parameter. The obtained results show that the protonation constants of the amino group (in log10 scale) are linearly related to the reciprocal of the dielectric constants of the water–organic solvent mixtures but this is not true for the protonation of the carboxylic acid group of the amino acids in both systems. Finally, the results are discussed in terms of the effect of solvent on the protonation and tautomeric constants.




Similar content being viewed by others
References
Nelson, D.L., Michael, M.: In: Principles of Biochemistry, 4th edn. W.H. Freeman, New York (2005)
Lehninger, A.L.: Principles of Biochemistry, 4th edn. W.H Freeman, New York (2005)
Yudkin, M.D., Offord, R.E.: Biochemistry. Houghton Mifflin Co., Oxford (1975)
D’Angelo, J.C., Collette, T.W.: A method for the measurement of site-specific tautomeric and zwitterionic microspecies equilibrium constants. Anal. Chem. 69, 1642–1650 (1997)
Hilal, S.H., Carreira, L.A., Melton, C.M., Baughman, G.L., Karickhoff, S.W.: Estimation of ionization constants of azo dyes and related aromatic amines: environmental implication. J. Phys. Org. Chem. 7, 122–141 (1994)
Zapala, L., Kalembkiewicz, J., Sitarz-Palczak, E.: Studies on equilibrium anthranilic acid in aqueous solutions and in two-phase systems: aromatic solvent–water. Biophys. Chem. 140, 91–98 (2009)
Mahali, K., Roy, S., Krishna Dolui, B.: Thermodynamic solvation of a series of homologous α-amino acids in non-aqueous mixture of ethylene-glycol and N,N-dimethyl formamide. J. Biophys. Chem. 2, 185–193 (2011)
Demirelli, H.: On the role of the solvent and substituent on the protonation equilibria of di-substituted anilines in dioxane–water mixed solvents. J. Solution Chem. 34, 1283–1295 (2005)
Sigel, H., Griesser, R.: Nucleoside 5′-triphosphates: self-association, acid-base, and metal ion-binding properties in solution. Chem. Soc. Rev. 34, 875–900 (2005)
Kauski, R., Murray, C.J.: Enzymichromism: determination of the dielectric properties of an enzyme active site. Tetrahedron Lett. 34, 3363–3366 (1993)
Li, Y.K., Kuliopulos, A., Mildvan, A.S., Talaly, P.: Environments and mechanistic roles of the tyrosine residues of DELTA 5-3-ketosteroid isomerase. Biochemistry 32, 1816–1824 (1993)
Roses, M., Buhvestov, U., Rafols, C., Rived, F., Bosch, E.: Solute–solvent and solvent–solvent interactions in binary solvent mixtures. A quantitative measurement of the enhancement of the water structure in 2-methylpropan-2-ol–water and propan-2-ol–water mixtures by solvatochromic indicators. J. Chem. Soc. Perkin Trans 2, 1341–1348 (1997)
Mancini, P.M.E., Terenzani, A., Adam, C., Vottero, L.R.: Solvent effects on aromatic nucleophilic substitution reactions. Part 7. Determination of the empirical polarity parameter ET(30) for dipolar hydrogen bond acceptor–co-solvent (chloroform or dichloromethane) mixtures. Kinetics of the reactions of halonitrobenzenes with aliphatic amines. J. Phys. Org. Chem. 10, 849–860 (1997)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry, 3rd edn. VCH, New York (2004)
Staszak, Z., Bartecki, A.: Influence of the bulk and donor–acceptor properties of solvent on ligand field spectra. Spectr. Lett. 22, 1193–1201 (1989)
Pehrsson, L., Ingman, F., Johansson, A.: Acid–base titrations by stepwise additions of equal volumes of titrant with special reference to automatic titrations—1. Theory, discussion of the Gran functions, the Hofstee method and two proposed methods for calculating equivalence volumes. Talanta 23, 769–774 (1976)
Gameiro, P., Reis, S., Lima, J.F.L.C., de Castro, B.: Calibration of pH glass electrodes by direct strong acid/strong base titrations under dilute conditions. Anal. Chim. Acta 405, 167–172 (2000)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the Hyperquad suite of programs. Talanta 43, 1739–1753 (1996)
Jimenez, J.A., Martinez, F.: Thermodynamic study of the solubility of acetaminophen in propylene glycol + water cosolvent mixtures. J. Braz. Chem. Soc. 17, 125–134 (2006)
Jimenez, J.A., Martinez, F.: Thermodynamic magnitudes of mixing and solvation of acetaminophen in ethanol + water cosolvent mixtures. Rev. Acad. Colomb. Cienc. 30, 87–99 (2006)
Faraji, M., Zare, K., Aghaei, H., Farajtabar, A., Asfari, Z., Gharib, F.: Complexation of p-sulphonato-calix[6]arene by glycine, glycyl-glycine, and glycyl-glycyl-glycine in aqueous solution. J. Solution Chem. 41, 2074–2081 (2012)
Koseoglu, F., Kilic, E., Dogan, A.: Studies on protonation constants and solvation of some α-amino acids in dioxan–water mixtures. Anal. Biochem. 277, 243–246 (2000)
Dogan, A., Koseoglu, F., Kilic, E.: Studies on macroscopic protonation constants of some α-amino acids in ethanol–water mixtures. Anal. Biochem. 309, 75–78 (2002)
Dogan, A., Kilic, E.: Potentiometric studies on protonation constants and solvation of some α-amino acid benzyl- and t-butyl-esters in ethanol–water mixtures. Turk. J. Chem. 29, 41–46 (2005)
Partanen, J.I., Juusola, P.M., Verraes, V.: Re-evaluation of the second thermodynamic dissociation constants of α-alanine, valine, and leucine using potentiometric data measured for aqueous potassium chloride solutions at 298.15 K. Can. J. Chem. 83, 46–52 (2005)
Izutsu, K.: Electrochemistry in Nonaqueous Solutions. Wiley, Weinheim (2002)
Garrido, G., Koort, E., Rafols, C., Bosch, E., Rodima, T., Leito, I., Roses, M.: Acid-base equilibria in nonpolar media. Absolute pKa scale of bases in tetrahydrofuran. J. Org. Chem. 71, 9062–9067 (2006)
Jabbari, M., Gharib, F.: Solute-solvent investigation effects on protonation equilibrium of some water-insoluble flavonoids. J. Solution Chem. 40, 561–574 (2011)
Barbosa, J., Barron, D., Beltran, J.L., Buti, S.: On the role of solvent in acid–base equilibria of diuretics in acetonitrile–water mixed solvents. Talanta 45, 817–827 (1998)
Barbosa, J., Toro, I., Sanz-Nebot, V.: Acid-base behavior of tripeptides in solvent used in liquid chromatography. Correlation between pK values and solvatochromic parameters of acetonitrile–water mixtures. Anal. Chim. Acta 347, 295–304 (1997)
Farajtabar, A., Gharib, F.: Spectral analysis of naringenin deprotonation in aqueous ethanol solutions. Chem. Pap 67, 538–545 (2013)
Taft, R., Abboud, J.L., Kamlet, M., Abraham, M.: Linear salvation energy relations. J. Solution Chem. 14, 153–186 (1985)
Jaberi, F., Gharib, F., Farajtabar, A.: Solute–solvent interaction effects on protonation and aggregation constants of TTMAPP in different aqueous solutions of methanol. J. Solution Chem. 42, 1559–1571 (2013)
Gharib, F., Abbaszadeh, M., Pousti, M.: Acid-base properties of adenosine 5′-monophosphate, guanosine 5′-monophosphate, and inosine 5′-monophosphate in aqueous solutions of methanol. Helv. Chim. Acta 96, 1134–1145 (2013)
Gharib, F., Feizabadi, M., Soltani, L.: Equilibrium studies of thallium(I) complexes with cytidine 5′-monophosphate in different aqueous solutions of methanol. J. Mol. Liq. 182, 64–69 (2013)
Jabbari, M., Gharib, F.: Solvent dependence on antioxidant activity of some water-insoluble flavonoids and their cerium(IV) complexes. J. Mol. Liq. 168, 36–41 (2012)
Taft, R.W., Abboud, J.L.M., Kamlet, M.J.: Linear solvation energy relationships. 28. An analysis of Swain’s solvent “acidity” and “basicity” scales. J. Org. Chem. 49, 2001–2005 (1984)
Kamlet, M.J., Abboud, J.L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. 23 A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 48, 2877–2887 (1983)
Buhvestov, U., Rived, F., Rafols, C., Bosch, E., Roses, M.: Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 7. Comparison of the enhancement of the water structure in alcohol–water mixtures measured by solvatochromic indicators. J. Phys. Org. Chem. 11, 185–192 (1998)
Barbosa, J., Barron, D., Buti, S.: Autoprotolysis constants and standardization of pH measurements in tetrahydrofuran–water mixtures. Electroanalysis 11, 627–631 (1999)
Puranik, S.M., Kumbharkhane, A.C., Mehrota, S.C.: The static permittivity of binary mixtures using an improved Bruggeman model. J. Mol. Liq. 59, 173–177 (1994)
Billo, E.J.: Excel for Chemists: A Comprehensive Guide. Wiley, Weinheim (2001)
Gharib, F.: Solvent effects on protonation and complexation of penicillamine and thallium(I) in different aqueous solutions of methanol. J. Chem. Eng. Data 55, 1547–1553 (2010)
Shamel, A., Saghiri, A., Jaberi, F., Farajtabar, A., Mofidi, F., Abedini Khorrami, S., Gharib, F.: Solvent effects on tautomeric and microscopic protonation constants of glycine in different aqueous solutions of methanol and ethanol. J. Solution Chem. 41, 1020–1032 (2012)
Dogan, A., Kilic, E.: Tautomeric and microscopic protonation equilibria of some α-amino acids. Anal. Biochem. 365, 7–13 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gharib, F., Nejhad, B.N. & Nouri, N. Tautomeric and Microscopic Protonation Constants of Alanine and Valine in Different Aqueous Solutions of Methanol and Tetrahydrofuran. J Solution Chem 44, 1655–1672 (2015). https://doi.org/10.1007/s10953-015-0369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0369-2