Abstract
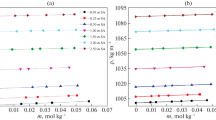
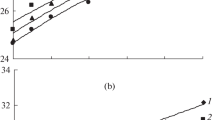
The physico-chemical property data for l-glutamic acid in aqueous NaCl solutions were obtained at 0, 0.5, 1.0 and 2.0 mol·kg−1 NaCl and amino acid molalities from 0 to 0.0669 mol·kg−1 between 293.15 and 323.15 K. The influence of NaCl addition on the volumetric and viscometric properties of the amino acid has been studied. From the experimental densities, the apparent molar volumes and transfer volumes of l-glutamic acid in aqueous electrolyte solutions have been calculated. The viscosity data in the studied domain of amino acid molalities have led to the evaluation of the Falkenhagen and Jones–Dole viscosity coefficients of l-glutamic acid in NaCl aqueous solutions. The results have been discussed in terms of interactions occurring in the systems.





Similar content being viewed by others
References
Badarayani, R., Kumar, A.: The mixing effect of glycylglycine with KCl, KBr, and Na2SO4 from volumetric and viscometric investigations at 298.15 K. J. Solution Chem. 33, 407–426 (2004)
Singh, S., Kishore, N.: Partial molar volumes of amino acids and peptides in aqueous salt solutions at 25 °C and a correlation with stability of protein in the presence of salts. J. Solution Chem. 32, 117–130 (2003)
Riyazuddeen, T., Khan, I.: Effect of KCl and KNO3 on partial molal volumes and partial molal compressibilities of some amino acids at different temperatures. Int. J. Thermophys. 30, 475–489 (2009)
Riyazuddeen, T., Altamash, T.: Ultrasonic velocities and densities of l-histidine or l-glutamic acid or l-tryptophan or glycylglycine + 2 mol·L−1 aqueous KCl or KNO3 solutions from 298.15 to 323.15 K. J. Chem. Eng. Data 54, 3133–3139 (2009)
Rodriguez, H., Soto, A., Arce, A., Khoshkbarchi, M.K.: Apparent molar volume, isentropic compressibility, refractive index, and viscosity of dl-alanine in aqueous NaCl solutions. J. Solution Chem. 32, 53–62 (2003)
Stefaniu, A., Iulian, O., Ciocirlan, O.: Mixing properties of some amino acids in aqueous electrolyte solutions. 19th International Congress of Chemical and Process Engineering CHISA, Praga, P3 172, pp. 677–678, ISBN 978-80-02-02247-3 (2010)
Ştefaniu, A., Iulian, O., Ciocîrlan, O.: Density, viscosity and refractive index of l-alanine and l-histidine in aqueous NaCl solutions at 298.15 K. Rev. Roum. Chim. 56, 869–874 (2011)
Munde, M.M., Kishore, N.: Volumetric properties of aqueous 2-chloroethanol solutions and volume of transfer of some amino acids and peptides from water to aqueous 2-chloroetahanol solutions. J. Solution Chem. 32, 791–802 (2003)
Banipal, T.S., Singh, K., Banipal, P.K.: Volumetric investigation on interactions of acidic/basic amino acids with sodium acetate, sodium propionate and sodium butyrate in aqueous solutions. J. Solution Chem. 36, 1635–1667 (2007)
Riyazuddeen, T., Afrin, S.: Viscosities of l-phenylalanine, l-leucine, l-glutamic acid, or l-proline + 2.0 mol·dm−3 aqueous NaCl or 2.0 mol·dm−3 aqueous NaNO3 solutions at T = 298.15 to 328.15 K. J. Chem. Eng. Data 55, 3282–3285 (2010)
Ali, A.: Shahjahan: Volumetric and viscometric behaviour of some amino acids and their group contributions in aqueous tetramethylammonium bromide at different temperatures. Z. Phys. Chem. 222, 1519–1532 (2008)
Jones, G., Dole, M.: The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 51, 2950–2964 (1929)
Millero, F.J., Surdo, A., Shin, C.: The apparent molal volumes and adiabatic compressibilities of aqueous amino acids at 25 °C. J. Phys. Chem. 82, 784–792 (1978)
Banipal, T.S., Kapoor, P.: Partial molal volumes and expansibilities of some amino acids in aqueous solutions. J. Indian Chem. Soc. 76, 431–437 (1999)
Jolicoeur, C., Riedl, B., Desrochers, D., Lemelin, L.L., Zamojska, R., Enea, O.: Solvation of amino acids residues in water and urea: Water mixtures: volumes and heat capacities of 20 amino acids in water and in 8 molar urea at 25 °C. J. Solution Chem. 15, 109–128 (1986)
Franks, F., Quickenden, M.A.J., Reid, D.S., Watson, B.: Calorimetric and volumetric studies of dilute aqueous solutions of cyclic ether derivatives. Trans. Faraday Soc. 66, 582–589 (1970)
Shahidi, F., Farrell, P.G., Edwards, J.T.: Partial molar volumes of organic compounds in water III. Carbohydrates. J. Solution Chem. 5, 807–816 (1976)
Shen, J.L., Li, Z.F., Wang, B.H., Zhang, Z.M.: Partial molar volumes of some amino acids and a peptide in water, DMSO, NaCl, and DMSO/NaCl aqueous solutions. J. Chem. Tehermodyn. 32, 805–819 (2000)
Zhang, H.L., Han, S.J.: Viscosity and density of water + sodium chloride + potassium chloride solutions at 298.15 K. J. Chem. Eng. Data 41, 516–520 (1996)
Motin, M.A.: Temperature and concentration dependence of apparent molar volumes and viscosities of NaCl, NH4Cl, CuCl2, CuSO4, and MgSO4 in pure water and water + urea mixtures. J. Chem. Eng. Data 49, 94–98 (2004)
Siddique, J.A., Naqvi, S.: Viscosity behavior of α-amino acids in acetate salt solutions at temperatures (303.15–323.15 K). Int. J. Thermophys. 33, 47–57 (2012)
Acknowledgments
The work has been funded by the Sectoral Operational Programme Human Resources Development 2007–2013 of the Romanian Ministry of Labour, Family and Social Protection through the Financial Agreement POSDRU/6/1.5/S/16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iulian, O., Stefaniu, A. Volumetric and Viscometric Analyses for l-Glutamic Acid in NaCl Aqueous Solutions at Different Temperatures. J Solution Chem 42, 676–689 (2013). https://doi.org/10.1007/s10953-013-9976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-9976-y