Abstract
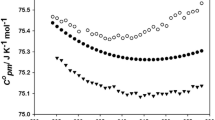
The crystal structure of n-undecylammonium bromide monohydrate was determined by X-ray crystallography. The crystal system of the compound is monoclinic, and the space group is P21/c. Molar enthalpies of dissolution of the compound at different concentrations m/(mol·kg−1) were measured with an isoperibol solution–reaction calorimeter at T = 298.15 K. According to the Pitzer’s electrolyte solution model, the molar enthalpy of dissolution of the compound at infinite dilution (\( \Updelta_{\text{sol}} H_{\text{m}}^{\infty } \)) and Pitzer parameters (\( \beta_{\text{MX}}^{(0)L} \) and \( \beta_{\text{MX}}^{(1)L} \)) were obtained. Values of the apparent relative molar enthalpies (\( {}^{\Upphi }L \)) of the title compound and relative partial molar enthalpies (\( \bar{L}_{2} \) and \( \bar{L}_{1} \)) of the solute and the solvent at different concentrations were derived from experimental values of the enthalpies of dissolution.




Similar content being viewed by others
References
Alkan, C., Sari, A., Karaipekli, A.: Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers. Manag. 52, 687–692 (2011)
Fang, G.Y., Li, H., Yang, F., Liu, X., Wu, S.M.: Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem. Eng. J. 153, 217–221 (2009)
Li, H., Liu, X., Fang, G.Y.: Preparation and characteristics of n-nonadecane/cement composites as thermal energy storage materials in buildings. Energy Build. 42, 1661–1665 (2010)
Rademeyer, M., Kruger, G.J., Billing, D.G.: Crystal structures and phase transitions of long-chain n-alkylammonium bromide monohydrates. Cryst. Eng. Comm. 11, 1926–1933 (2009)
Kruger, G.J., Rademeyera, M., Billingb, D.G.: n-Undecylammonium bromide monohydrate. Acta Cryst. E59, o480–o482 (2003)
Dan, W.Y., Di, Y.Y., Xin, C.L., Kong, Y.X., Tan, Z.C.: Low-temperature heat capacities and standard molar enthalpy of formation of ethylenediammonium tetrachlorocobaltate(II) chloride (H3NCH2CH2NH3)2[CoCl4]Cl2(s). J. Chem. Eng. Data 55, 3010–3016 (2010)
Di, Y.Y., Yang, W.W., Kong, Y.X., Shi, Q., Tan, Z.C.: Low–temperature heat capacities and standard molar enthalpy of formation of L-3-(3,4-dihydroxyphenyl) alanine (C9H11NO4). J. Chem. Eng. Data 53, 900–904 (2008)
Yin, H.D., Chen, S.W., Li, L.W., Wang, D.Q.: Synthesis, characterization and crystal structures of the organotin(IV) compounds with the Schiff base ligands of pyruvic acid thiophene-2-carboxylic hydrazone and salicylaldehyde thiophene-2-carboxylic hydrazone. Inorg. Chim. Acta 360, 2215–2223 (2007)
Yang, W.W., Di, Y.Y., Li, J., Kong, Y.X.: Thermochemistry on ephedrine hydrochloride and N-methylephedrine hydrochloride. J. Chem. Thermodyn. 41, 945–950 (2009)
Liu, Y.P., Di, Y.Y., He, D.H., Kong, Y.X., Yang, W.W., Dan, W.Y.: Lattice potential energy and thermochemical properties of ethylenediamine dihydrochloride (C2H10N2Cl2). J. Chem. Thermodyn. 42, 513–517 (2010)
Rychly, R., Pekarek, V.: The use of potassium chloride and tris-(hydroxymethyl) aminomethane as standard substances for solution calorimetry. J. Chem. Thermodyn. 9, 391–396 (1977)
Silvester, L.F., Pitzer, K.S.: Thermodynamics of electrolytes VIII. High-temperature properties, including enthalpy and heat capacity, with application to sodium chloride. J. Phys. Chem. 81, 1822–1828 (1977)
Pitzer, K.S. (ed.): Activity coefficients in electrolyte solutions, 2nd edn. CRC, Boca Raton (1991)
Acknowledgments
This work was financially supported by the National Natural Science Foundations of China under the contracts NSFC No. 20673050 and 20973089.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, LJ., Di, YY. & Dou, JM. Thermochemical Properties of n-Undecylammonium Bromide Monohydrate C11H28BrNO(s). J Solution Chem 42, 52–59 (2013). https://doi.org/10.1007/s10953-013-9954-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-9954-4