Abstract
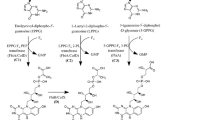
Methanogens play a critical role in carbon cycling and contain a number of intriguing biosynthetic pathways. One unusual cofactor found in methanogenic and sulfate reducing archaea is Factor 420 (F420), which can be interconverted between its reduced and oxidized forms by the F420H2:NADP+ oxidoreductase (Fno) through hydride transfer mechanisms. Here, we report an optimized expression and purification method for recombinant Fno derived from the extreme thermophile Archeoglobus fulgidus. An expression vector that is codon-optimized for heterologous expression in Escherichia coli, modified growth conditions, and a modified purification protocol involving a key polyethyleneimine precipitation step results in a highly purified, homogeneous preparation of Fno that displays high catalytic activity with a truncated F420 analog. This method should accelerate studies on how Fno uses the unusual F420 cofactor during catalysis.




Similar content being viewed by others
Abbreviations
- Fno:
-
F420H2:NADP+ oxidoreductase
- NADP+ :
-
Nicotinamide adenine dinucleotide phosphate
- E. coli :
-
Escherichia coli
- Archeoglobus fulgidus :
-
A. fulgidus
- IPTG:
-
Isopropyl β-D-1-thiogalactopyranoside
- FO:
-
F420 cofactor precursor
- LB:
-
Luria Bertani broth
- Tris:
-
Tris(hydroxymethyl)aminomethane
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- SDS–PAGE:
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- k cat :
-
Michaelis–Menten catalytic rate constant (turnover number)
References
Jacobson F, Walsh C (1984) Properties of 7,8-didemethyl-8-hydroxy-5-deazaflavins relevant to redox coenzyme function in methanogen metabolism. Biochemistry 23:979–988
Costa KC, Leigh JA (2014) Metabolic versatility in methanogens. Curr Opin Biotechnol 29:70–75
Ersahin EM, Gomec CY, Dereli RK, Arikan O, Ozturk I (2011) Biomethane production as an alternative bioenergy source from codigersters treating municipal sludge and organic fraction of municipal solid wastes. J Biomed Biotechnol 2011:1–8
Osumah T, Le CQ, Nguyen TD, Johnson-Winters K (2013) The biochemistry of F420 cofactor biosynthesis. In: Mandal SS (ed) Recent trends in gene expression and regulation. Nova Science Publisher, New York, pp 75–106
Joseph E, Le CQ, Nguyen T, Hossain MS, Oyugi M, Foss FW Jr, Johnson-Winters K (2015) Evidence of negative cooperativity and half-site reactivity within an F420-dependent enzyme: a kinetic analysis of F420H2:NADP+ oxidoreductase Biochemistry; to be submitted for publication
Warkentin E, Mamat B, Sordel-Klippert M, Wicke M, Thauer RK, Iwata M, Iwata S, Ermler U, Shima S (2001) Structures of F420H2:NADP+ oxidoreductase with and without its substrates bound. EMBO J 20:6561–6569
Elias DA, Juck DF, Berry KA, Sparl R (2000) Purification of the NADP+: F420 oxidoreductase of Methanosphaera stadtmanae Can. J Microbiol 46:998–1003
Eirich LD, Dugger RS (1984) Purification and properties of an F420-dependent NADP reductase from Methanobacterium thermoautotrophicum Biochim. Biophys. Acta 802:454–458
Yamazaki S, Tsai L (1980) Purification and properties of 8-hydroxy-5-deazaflavin-dependent NADP+ reductase from Methanococcus vannielii. J Biol Chem 255:6462–6465
Berk H, Thauer RK (1997) Function of coenzyme F420-dependent NADP reductase in methanogenic archaea containing an NADP-dependent alcohol dehydrogenase. Arch Microbiol 168:396–402
Kunow J, Schwoërer B, Stetter KO (1993) Thauer RKA F420-dependent NADP reductase in the extremely thermophilic sulfate-reducing Archaeoglobus fulgidus. Arch Microbiol. 160:199–205
DeWit LEA, Eker APM (1987) 8-Hydroxy-5-deazaflavin-dependent electron transfer in the extreme halophile Halobacterium cutirubrum FEMS. Microbiol Lett. 48:121–125
Eker APM, Hessels JKC, Meerwaldt R (1989) Characterization of an 8-hydroxy-5-deazaflavin: NADPH oxidoreductase from Streptomyces griseus. Biochim Biophys Acta 990:80–86
Murzin AG, Brenner SE, Hubbard T, Chothia C (1995) SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol 247:536–540
Berk H, Thauer RK (1998) F420H2:NADP oxidoreductase from Methanobacterium thermoautotrophicum: identification of the encoding gene via functional overexpression in Escherichia coli. FEBS Lett 438:124–126
Hossain MS, Le CQ, Joseph E, Nguyen TQ, Johnson-Winters K, Foss FW Jr (2015) Convenient synthesis of deazaflavin cofactor FO and its activity in F420-dependent NADP reductase. Org Biomol Chem 13:5082–5085
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lambin P, Fine JM (1979) Molecular weight estimation of proteins by electrophoresis in linear polyacrylamide gradient gels in the absence of denaturing agents. Anal Biochem. 98:160–168
Fairbanks G, Steck TL, Wallach DFH (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606–2617
Wenrich BR, Trumbo TA (2012) Interaction of nucleic acids with coomassie blue G-250 in the bradford assay. Anal Biochem. 428:93–95
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, New Jersey, pp 571–607
Dumon-Seignovert L, Cariot G, Vuilard L (2004) The toxicity of recombinant proteins in Escherichia coli: a comparison of overexpression in BL21(DE3), C41(DE3), and C43(DE3) protein. Exp Purif 37:203–206
Burgess RR (2009) Protein precipitation techniques. In: Burgess RR, Deutscher MP (eds) Methods in enzymology. Academic Press, Massachusetts, pp 331–342
Acknowledgments
This work was supported by the National Science Foundation, Grant number, 1120837 (to KJW). We also thank Drs. John H. Enemark, Graham R. Moran, Frank W. Foss, Richard Timmons and Walter Fast for helpful discussions. Figure 4 (right figure, steady-state kinetics plot with FO) was reproduced from the following reference. Hossain, M. S.; Le, C. Q.; Joseph, E.; Nguyen, T. Q.; Johnson-Winters, K.; Foss, F. W., Jr. Convenient synthesis of deazaflavin cofactor FO and its activity in F420-dependent NADP reductase. Org. Biomol. Chem, 13, 5082–5085. Reproduced by permission of The Royal Society of Chemistry.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le, C.Q., Joseph, E., Nguyen, T. et al. Optimization of Expression and Purification of Recombinant Archeoglobus fulgidus F420H2:NADP+ Oxidoreductase, an F420 Cofactor Dependent Enzyme. Protein J 34, 391–397 (2015). https://doi.org/10.1007/s10930-015-9633-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-015-9633-y