Abstract
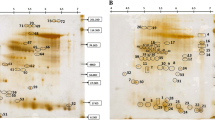
Rice is the most important crop consumed all over the world. In Brazil, irrigated rice covers 50 % of the rice producing area and is responsible for 75 % of the national production. Upland rice covers most of the remaining area, and is therefore, a very important production system in the country. In the present study, we have used the drought tolerant upland rice variety Três Meses Antigo to investigate the proteomic changes that occur during drought stress. Plants were submitted to drought by the reposition of 50 % of the water lost daily. Twenty days after the beginning of the drought stress period, leaves were harvested and used for protein extraction. The 2D maps obtained from treated and control plants revealed 408 reproducible spots, 44 of which were identified by mass spectrometry, including 15 differential proteins. Several unaltered proteins were also identified (39 spots) and were mainly involved in photosynthesis. Taken together, the results obtained suggest that the tolerant upland rice up-regulates anti-oxidant and energy production related proteins in order to cope with water deficit.


Similar content being viewed by others
Abbreviations
- PVC:
-
Polyvinyl chloride
- ABA:
-
Abscisic acid
- 3MA:
-
Três meses antigo
- CHAPS-3:
-
[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DTT:
-
Dithiothreitol
- IPG:
-
Immobilized pH gradient
- SDS–PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionization-time-of-flight
- MS:
-
Mass spectrometry
- SNAP:
-
Sophisticated numerical annotation procedure
References
Aselmann I, Crutzen PJ (1989) Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J Atmos Chem 8:307–358
Ali GM, Komatsu S (2006) Proteomic analysis of rice leaf sheath during drought stress. J Proteome Res 5:396–403
Komatsu S (2005) Rice proteome database: a step toward functional analysis of the rice genome. Plant Mol Biol 59:179–190
Lu T, Lu G, Fan D, Zhu C, Li W, Zhao Q, Feng Q, Zhao Y, Guo Y, Huang X, Han B (2010) Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq. Genome Res 20:1238–1249
Helmy M, Tomita M, Ishihama Y (2011) OryzaPG-DB: rice proteome database based on shotgun proteogenomics. BMC Plant Biol 11:63
Yang Y, Dai L, Xia H, Zhu K, Liu H, Chen K (2013) Protein profile of rice (Oryza sativa) seeds. Gen Mol Biol 36:87–92
Agrawal GK, Rakwal R (2011) Rice proteomics: a move toward expanded proteome coverage to comparative and functional proteomics uncovers the mysteries of rice and plant biology. Proteomics 11:1630–1649
Singh R, Jwa NS (2013) Understanding the responses of rice to environmental stress using proteomics. J Proteome Res 11:4652–4669
Mirzaei M, Soltani N, Sarhadi E, Pascovici D, Keighley T, Salekdeh GH, Haynes PA, Atwell BJ (2012) Shotgun proteomic analysis of long-distance drought signaling in rice roots. J Proteome Res 11:348–358
Kosova K, Vitamvas P, Prasil IT, Renaut J (2011) Plant proteome changes under abiotic stress—contribution of proteomics studies to understanding plant stress response. J Proteomics 74:1301–1322
Liu JX, Bennett J (2011) Reversible and irreversible drought-induced changes in the anther proteome of rice (Oryza sativa L.) genotypes IR64 and Moroberekan. Mol Plant 4:59–69
Mushtaq R, Katiyar S, Bennett J (2008) Proteomic analysis of drought stress-responsive proteins in rice endosperm affecting grain quality. J Crop Sci Biotech 4:227–232
Mirzaei M, Soltani N, Sarhadi E, Pascovici D, Keighley T, Salekdeh GH, Haynes PA, Atwell BJ (2012) Shotgun proteomic analysis of long-distance drought signaling in rice roots. J Proteome Res 1:348–358
Rabello AR, Guimaraes CM, Rangel PH, da Silva FR, Seixas D, de Souza E, Brasileiro AC, Spehar CR, Ferreira ME, Mehta A (2008) Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genom 9:485
Huang W, Bi T, Sun W (2010) Comparative analysis of panicle proteomes of two upland rice varieties upon hyper-osmotic stress. Front Biol 5:546–555
Guimarães C, Stone L, Rangel P, Ferreira M (2006) In: 2º Congresso Brasileiro da Cadeia Produtiva de Arroz/VIII. Resitência à seca: I Avaliação de genótipos de arroz de terras altas, com divergência genotípica, em condições controlada. Reunião Nacional de Pesquisa de Arroz
Fischer R, Maurer R (1978) Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust J Agric Res 29:897–912
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–858
Ke Y, Han G, He H, Li J (2009) Differential regulation of proteins and phosphoproteins in rice under drought stress. Biochem Biophys Res Commun 379:133–138
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Portis AR (1992) Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol Plant Mol Biol 43:415–437
Campbell WJ, Ogren WL (1995) Rubisco activase activity in spinach leaf extracts. Plant Cell Physiol 36:215–220
Ferreira VM, Magalhães PC, Durães FOM, Oliveira LEMD, Purcino AÁC (2002) Metabolismo do nitrogênio associado à deficiência hídrica e sua recuperação em genótipos de milho. Ciência Rural 32:13–17
Salekdeh GH, Siopongco J, Wade LJ, Ghareyazie B, Bennett J (2002) Proteomic analysis of rice leaves during drought stress and recovery. Proteomics 2:1131–1145
Kumari S, Nee Sabharwal VP, Kushwaha HR, Sopory SK, Singla-Pareek SL, Pareek A (2009) Transcriptome map for seedling stage specific salinity stress response indicates a specific set of genes as candidate for saline tolerance in Oryza sativa L. Funct Integr Genomics 9:109–123
Wang H, Zhang H, Li Z (2007) Analysis of gene expression profile induced by water stress in upland rice (Oryza sativa L. var. IRAT109) seedlings using subtractive expressed sequence tags library. J Int Plant Biol 49:1455–1463
Berna A, Bernier F (1999) Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol Biol 39:539–549
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Eniron Exp Bot 49:69–76
Mittal R, Dubey RS (1991) Behaviour of peroxidases in rice: changes in enzyme activity and isoforms in relation to salt tolerance. Plant Physiol Biochem 29:31–40
Nelson CJ, Hegeman AD, Harms AC, Sussman MR (2006) A quantitative analysis of Arabidopsis plasma membrane using trypsin-catalyzed (18) O labeling. Mol Cell Proteomics 5:1382–1395
Valot B, Negroni L, Zivy M, Gianinazzi S, Dumas-Gaudot E (2006) A mass spectrometric approach to identify arbuscular mycorrhiza-related proteins in root plasma membrane fractions. Proteomics 6:145–155
Lefebvre B, Furt F, Hartmann MA, Michaelson LV, Carde JP, Sargueil-Boiron F, Rossignol M, Napier JA, Cullimore J, Bessoule JJ, Mongrand S (2007) Characterization of lipid rafts from medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft-associated redox system. Plant Physiol 144:402–418
Hazen SP, Pathan MS, Sanchez A, Baxter I, Dunn M, Estes B, Chang HS, Zhu T, Kreps JA, Nguyen HT (2005) Expression profiling of rice segregating for drought tolerance QTLs using a rice genome array. Funct Integr Genomics 5:104–116
Wang D, Pan Y, Zhao X, Zhu L, Fu B, Li Z (2011) Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom 12:149
Oztur ZN, Talame V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ (2002) Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol Biol 48:551–573
Zhang J, Peng Y, Guo Z (2008) Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res 18:508–521
Zhu XF, Chen XW, Li XB, Qian Q, Huang DN, Zhu LH, Zhai WX (2002) Genetic mapping of T-DNA integration sites in Xa21 transgenic rice. Yi Chuan Xue Bao 29:880–886
Düaiucl H, Alleweldt G (1973) Der lahresgang der Abscisinsäure in vegetativen Organen von Reben. Vitis 12:26–32
Broquedis M (1987) L’acide abscissique et l’abscissate de B-D-glucopyranose dans le développement des baies de raisin, la germination des pépins et la formation des racines sur les boutures de Vigne. Vittis 28:121–135
Düring H, Bachmann O (1975) Abscisic acid analysis in Vitis vinifera in the period of endogenous bud dormancy by high pressure liquid chromatography. Physiol Plantarum 34:201–203
During H (1992) Gas exchange of grapcvines leaves as affected by soil factors. In: Simposium international sur la physiologie de la vign. In Comptes Rendas 295–298
Broquedis M, Bouard L (1989) L’acide abscissique dans différents aspects de la physiologie de la Vigne. Connaissanee de la Vigne et du Vin—Aspects Actuels de la Viticulture. In Hors Série 89–94
Tabaeizadeh Z (1998) Drought-induced responses in plant cells. Int Rev Cytol 182:193–247
Dooki AD, Mayer-Posner FJ, Askari H, Zaiee AA, Salekdeh GH (2006) Proteomic responses of rice young panicles to salinity. Proteomics 6:6498–6507
Imin N, Kerim T, Rolfe BG, Weinman JJ (2004) Effect of early cold stress on the maturation of rice anthers. Proteomics 4:1873–1882
Liska AJ, Shevchenko A, Pick U, Katz A (2004) Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol 136:2806–2817
Yan SP, Zhang QY, Tang ZC, Su WA, Sun WN (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteomics 5:484–496
Acknowledgments
This research was supported by Embrapa, CNPq and Embrapa Recursos Genéticos e Biotecnologia. The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabello, F.R., Villeth, G.R.C., Rabello, A.R. et al. Proteomic Analysis of Upland Rice (Oryza sativa L.) Exposed to Intermittent Water Deficit. Protein J 33, 221–230 (2014). https://doi.org/10.1007/s10930-014-9554-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-014-9554-1