Abstract
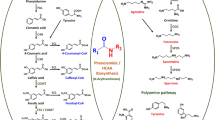
A NAD(P) reductase-like protein with a molecular mass of 34.146 ± 34 Da was purified to homogeneity from the appendix of the inflorescence of the Sauromatum guttatum. On-line liquid chromatography/electrospray ionization-mass spectrometry was used to isolate and quantify the protein. For the identification of the protein, liquid chromatography/electrospray ionization-tandem mass spectrometry analysis of tryptic digests of the protein was carried out. The acquired mass spectra were used for database searching, which led to the identification of a single tryptic peptide. The 12 amino acid tryptic peptide (FLPSEFGNDVDR) was found to be identical to amino acid residues at the positions 108–120 of isoflavone reductase in the Arabidopsis genome. A BLAST search identified this sequence region as unique and specific to a class of NAD(P)-dependent reductases involved in phenylpropanoid biosynthesis. Edman degradation revealed that the protein was N-terminally blocked. The amount of the protein (termed RL, NAD(P) reductase-like protein) increased 60-fold from D-4 (4 days before inflorescence-opening, designated as D-day) to D-Day, and declined the following day, when heat-production ceased. When salicylic acid, the endogenous trigger of heat-production in the Sauromatum appendix, was applied to premature appendices, a fivefold decrease in the amount of RL was detected in the treated section relative to the non-treated section. About 40 % of RL was found in the cytoplasm. Another 30 % was detected in Percoll-purified mitochondria and the rest, about 30 % was associated with a low speed centrifugation pellet due to nuclei and amyloplast localization. RL was also found in other thermogenic plants and detected in Arabidopsis leaves. The function of RL in thermogenic and non-thermogenic plants requires further investigation.





Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- D-Day:
-
The day of inflorescence opening and heat-production
- IP:
-
Isopropanol
- ESI:
-
Electrospray ionization
- LC:
-
Liquid chromatography
- MaxEnt:
-
Maximum entropy software for deconvolution of multiply charged electrospray envelopes
- MS:
-
Mass spectrometry
- NIPIA:
-
N-isopropyl iodoacetamide
- OBs:
-
Oil bodies
- ODs:
-
Osmiophilic deposits
- PVP:
-
Polyvinylpyrrolidone
- RL:
-
NAD(P) reductase-like protein
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase oxygenase
- SA:
-
Salicylic acid
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- TIC:
-
Total ion current
- TFA:
-
Trifluoroacetic acid
- TPCK:
-
L-1-tosylamido-2-phenylethyl chloromethyl ketone
References
Akashi T, Koshimizu S, Aoki T, Ayabe S (2006) FEBS Lett 580:5666–5670
Ayala G, Nascimento A, Gómez-Puyou A, Darszon A (1985) Biochem Biophys Acta 810:115–122
Chertov O, Simpson JT, Biragyn A, Conrads TP, Veenstra TD, Fisher RJ (2005) Expert Rev Proteomics 2:139–145
D’Andréa S, Jolivet P, Boulard C, Larré C, Froissard M, Chardot T (2007) J Agric Food Chem 55:10008–10015
Darszon A, Gómez-Puyou A (1982) Eur J Biochem 12:427–433
Dinova-Kostova AT, Gang DR, Davin LB, Bedgar DL, Chu A, Lewis NG (1996) J Biol Chem 271:29473–29482
Dixon RA, Paiva NL (1995) Plant Cell 7:1085–1097
Elthon TE, McIntosh L (1986) Plant Physiol 82:1–6
Fahn A (1979) Secretory tissues in plants. Academic Press, New York
Ferre JL, Austin MB, Stewart C, Noel JP (2008) Plant Physiol Biochem 46:356–370
Gang DR, Kasahara H, Xia Z-Q, Mijnsbrugge KV, Bauw G, Boerjan W, Van Montagu M, Davin LB, Lewis NG (1999) J Biol Chem 274:7516–7527
Higdon R, Kolker E (2007) Bioinformatics 23:277–280
Horn PJ, Ledbetter NR, James CN, Hoffman WD, Case CR, Verbeck GF, Chapman KD (2010) J Biol Chem 286:3298–3306
Koeduka T, Fridman E, Gang DR, Vassão DG, Jackson BL, Kish CM, Oova I, Spassova SM, Lewis NG, Noel JP, Baiga TJ, Dudareva N, Pichersky E (2006) Proc Nat Acad Sci USA 26:10128–10133
Lamb HK, Leslie K, Dodds AL, Nutley M, Cooper A, Johnson C, Thompson P, Stammers DK, Hawkins AR (2003) J Biol Chem 278:32107–32114
Lamb HK, Stammers DK, Hawkins AR (2008) Sci Signal 133:pe38
Laemmli UK (1970) Nature 227:680–685
Link AJ, Eng J, Schieltz DM, Carmack E, Mize GM, Morris DR, Garvik BM, Yates JR (1999) Nature Biotech 17:676–682
Meeuse BJD (1985) In: Palmer JM (ed) The physiology and biochemistry of plant respiration. Cambridge University Press, Cambridge, pp 47–58
Moore BD (2004) Plant Sci 9:221–228
Murphy DJ, Vance J (1999) Trends Biochem Sci 24:109–115
Nakatsudo T, Mizutani M, Suzuki S, Hattori T, Umezawa T (2008) J Biol Chem 283:15550–15557
Núñez-Corcuera B, Serafimidis J, Arias-Palomo E, Rivera-Calzada A, Suarez T (2008) Dev Biol 321:331–342
Polevoda B, Sherman F (2002) Genome Biol 3:REVIEWS0006
Quail PH (1979) Ann Rev Plant Physiol 30:425–484
Raskin I, Ehmann A, Melander WR, Meeuse BJD (1987) Science 237:1601–1602
Shiojiri H, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J (2006) Proc Nat Acad Sci USA 103:16672–16676
Siloto RM, Findlay K, Lopez-Villaobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006) Plant Cell 18:1961–1974
Skubatz H, Meeuse BJD, Bendich AJ (1989) Plant Physiol 91:530–535
Skubatz H, Nelson TA, Meeuse BJD, Bendich AJ (1991) Plant Physiol 95:1084–1088
Skubatz H, Kunkel DD, Meeuse BJD (1993) Sex Plant Reprod 6:153–170
Skubatz H, Kunkel DD, Patt J, Howald WN, Rothman T, Meeuse BJD (1995) Proc Nat Acad Sci USA 92:10084–10088
Skubatz H, Kunkel DD, Howald WN, Trenkle R, Mookherjee B (1996) New Phytol 134:631–640
Skubatz H, Orellana MV, Yablonka-Reuveni Z (2000) Histochem J 32:467–474
Tang W (1987) Bot Gazette 148:165–174
Tikunov YM, de Vos RCH, González Paramás AM, Hall RD, Bovy AG (2010) Plant Physiol 152:55–70
Uritani I, Asahi T (1980) The biochemistry of plants, vol 2. Academic Press, New York, pp 463–483
Vander Mijnsbrugge K, Beeckman H, De Rycke R, Van Montagu M, Engler G (2000) Planta 211:502–509
Vogel S (1989) In: Renner S (ed) The role of scent glands in pollination. Amerind, New Delhi
Wang J, Dudareva N, Bhakta S, Raguso RA, Pichersky E (1997) Plant Physiol 114:213–221
Wang X, He X, Lin J, Shao H, Chang Z, Dixon RA (2006) J Mol Biol 358:1341–1352
Waridel P, Frank A, Thomass H, Surendranath V, Sunyaev S, Pevzner P, Schevchenko A (2007) Proteomics 7:2318–2329
Acknowledgments
We thank the University of Washington, Department of Medicinal Chemistry, Mass Spectrometry Center for their assistance in the isolation, purification and quantification of RL; and the Harvard Medical School, Department of Genetics, Biopolymers Facility for their assistance in the identification of the tryptic peptide; and the Biomolecular Research Facility at the University of Virginia for their Edman degradation analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skubatz, H., Howald, W.N. Purification of a NAD(P) Reductase-Like Protein from the Thermogenic Appendix of the Sauromatum guttatum Inflorescence. Protein J 32, 197–207 (2013). https://doi.org/10.1007/s10930-013-9472-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9472-7