Abstract
One critical approach to preclinical evaluation of anti-tuberculosis (anti-TB) drugs is the study of correlations between drug exposure and efficacy in animal TB infection models. While such pharmacokinetic/pharmacodynamic (PK/PD) studies are useful for the identification of optimal clinical dosing regimens, they are resource intensive and are not routinely performed. A mathematical model capable of simulating the PK/PD properties of drug therapy for experimental TB offers a way to mitigate some of the practical obstacles to determining the PK/PD index that best correlates with efficacy. Here, we present a preliminary physiologically based PK/PD model of rifampin therapy in a mouse TB infection model. The computational framework integrates whole-body rifampin PKs, cell population dynamics for the host immune response to Mycobacterium tuberculosis infection, drug-bacteria interactions, and a Bayesian method for parameter estimation. As an initial application, we calibrated the model to a set of available rifampin PK/PD data and simulated a separate dose fractionation experiment for bacterial killing kinetics in the lungs of TB-infected mice. The simulation results qualitatively agreed with the experimentally observed PK/PD correlations, including the identification of area under the concentration-time curve as best correlating with efficacy. This single-drug framework is aimed toward extension to multiple anti-TB drugs in order to facilitate development of optimal combination regimens.




Similar content being viewed by others
References
Laurenzi M, Ginsberg A, Spigelman M (2007) Challenges associated with current and future TB treatment. Infect Disord Drug Targets 7(2):105–119
Spigelman M, Woosley R, Gheuens J (2010) New initiative speeds tuberculosis drug development: novel drug regimens become possible in years, not decades. Int J Tuberc Lung Dis 14(6):663–664
Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH (2008) Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 52(4):1522–1524
Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL (2011) Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55(12):5485–5492
Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL (2012) Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56(6):3114–3120
Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK (2012) 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380(9846):986–993
Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J (2011) Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 14(2):107–117
Chang KC, Leung CC, Grosset J, Yew WW (2011) Treatment of tuberculosis and optimal dosing schedules. Thorax 66(11):997–1007
De Groote MA, Gruppo V, Woolhiser LK, Orme IM, Gilliland JC, Lenaerts AJ (2012) Importance of confirming data on the in vivo efficacy of novel antibacterial drug regimens against various strains of Mycobacterium tuberculosis. Antimicrob Agents Chemother 56(2):731–738
Grosset J, Almeida D, Converse PJ, Tyagi S, Li SY, Ammerman NC, Pym AS, Wallengren K, Hafner R, Lalloo U, Swindells S, Bishai WR (2012) Modeling early bactericidal activity in murine tuberculosis provides insights into the activity of isoniazid and pyrazinamide. Proc Natl Acad Sci USA 109(37):15,001–15,005
Ginsberg A (2011) The TB Alliance: overcoming challenges to chart the future course of TB drug development. Future Med Chem 3(10):1247–1252
Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V (2003) Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47(7):2118–2124
Jayaram R, Shandil RK, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Kantharaj E, Balasubramanian V (2004) Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 48(8):2951–2957
Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Balasubramanian V (2007) Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 51(2):576–582
Pasipanodya J, Gumbo T (2011) An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 55(1):24–34
Ahmad Z, Peloquin CA, Singh RP, Derendorf H, Tyagi S, Ginsberg A, Grosset JH, Nuermberger EL (2011) PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob Agents Chemother 55(1):239–245
Tasneen R, Williams K, Amoabeng O, Minkowski A, Mdluli KE, Upton AM, Nuermberger EL (2015) Contribution of the nitroimidazoles PA-824 and TBA-354 to the activity of novel regimens in murine models of tuberculosis. Antimicrob Agents Chemother 59(1):129–135
Meibohm B, Derendorf H (1997) Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther 35(10):401–413
Katsube T, Yamano Y, Yano Y (2008) Pharmacokinetic-pharmacodynamic modeling and simulation for in vivo bactericidal effect in murine infection model. J Pharm Sci 97(4):1606–1614
Katsube T, Yano Y, Yamano Y, Munekage T, Kuroda N, Takano M (2008) Pharmacokinetic-pharmacodynamic modeling and simulation for bactericidal effect in an in vitro dynamic model. J Pharm Sci 97(9):4108–4117
Nielsen EI, Cars O, Friberg LE (2011) Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 55(10):4619–4630
Tan YM, Clewell H, Campbell J, Andersen M (2011) Evaluating pharmacokinetic and pharmacodynamic interactions with computational models in supporting cumulative risk assessment. Int J Environ Res Public Health 8(5):1613–1630
Lyons MA, Reisfeld B, Yang RS, Lenaerts AJ (2013) A physiologically based pharmacokinetic model of rifampin in mice. Antimicrob Agents Chemother 57(4):1763–1771
Friedman A, Turner J, Szomolay B (2008) A model on the influence of age on immunity to infection with Mycobacterium tuberculosis. Exp Gerontol 43(4):275–285
Vaddady PK, Lee RE, Meibohm B (2010) In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: focus on TB. Future Med Chem 2(8):1355–1369
Charles River Laboratories Inc (2006) Charles River Laboratories Research Models & Services. http://www.criver.com
Gibaldi M, Perrier D (1982) Pharmacokinetics. Marcel Dekker Inc, New York
Cheng S, Bois FY (2011) A mechanistic modeling framework for predicting metabolic interactions in complex mixtures. Environ Health Perspect 119(12):1712–1718
Bernillon P, Bois FY (2000) Statistical issues in toxicokinetic modeling: a Bayesian perspective. Environ Health Perspect 108(Suppl 5):883–893
Frantz J (2012) G3data, version 1.5.2, Software. https://github.com/pn2200/g3data.git
Bauer B, Reynolds M (2008) Recovering data from scanned graphs: performance of Frantz’s g3data software. Behav Res Methods 40(3):858–868
Chiu WA, Okino MS, Evans MV (2009) Characterizing uncertainty and population variability in the toxicokinetics of trichloroethylene and metabolites in mice, rats, and humans using an updated database, physiologically based pharmacokinetic (PBPK) model, and Bayesian approach. Toxicol Appl Pharmacol 241(1):36–60
Gelman A, Carlin JB, Stern HS, Rubin DB (2003) Bayesian data analysis, 2nd edn. Chapman and Hall/CRC, Boca Raton
Bois FY, Maszle DR (1997) MCSim: a Monte Carlo simulation program. J Stat Softw 2(i09):1–60
R Core Team (2012) R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Plummer M, Best N, Cowles K, Vines K (2006) CODA: convergence diagnosis and output analysis for MCMC. R News 6(1):7–11
van der Graff PH, Benson N (2011) Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm Res 28(7):1460–1464
Goutelle S, Bourguignon L, Jelliffe RW, Conte JE, Maire P (2011) Mathematical modeling of pulmonary tuberculosis therapy: Insights from a prototype model with rifampin. J Theor Biol 282(1):80–92
Magombedze G, Garira W, Mwenje E (2006) Mathematical modeling of chemotherapy of human TB infection. J Biol Syst 14:509–553
Yang RS, El-Masri HA, Thomas RS, Dobrev ID, Dennison JE, Bae DS, Campain JA, Liao KH, Reisfeld B, Andersen ME, Mumtaz M (2004) Chemical mixture toxicology: from descriptive to mechanistic, and going on to in silico toxicology. Environ Toxicol Pharmacol 18(2):65–81
Mumtaz M, Fisher J, Blount B, Ruiz P (2012) Application of physiologically based pharmacokinetic models in chemical risk assessment. J Toxicol 904:603
Baciewicz AM, Chrisman CR, Finch CK, Self TH (2013) Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin 29(1):1–12
Raybon JJ, Pray D, Morgan DG, Zoeckler M, Zheng M, Sinz M, Kim S (2011) Pharmacokinetic-pharmacodynamic modeling of rifampicin-mediated Cyp3a11 induction in steroid and xenobiotic X receptor humanized mice. J Pharmacol Exp Ther 337(1):75–82
Dooley KE, Flexner C, Andrade AS (2008) Drug interactions involving combination antiretroviral therapy and other anti-infective agents: repercussions for resource-limited countries. J Infect Dis 198(7):948–961
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Gonzalez-Juarrero M (2012) Immunity to TB and targets for immunotherapy. Immunotherapy 4(2):187–199
Gonzalez-Juarrero M, Woolhiser LK, Brooks E, DeGroote MA, Lenaerts AJ (2012) Mouse model for efficacy testing of antituberculosis agents via intrapulmonary delivery. Antimicrob Agents Chemother 56(7):3957–3959
Srivastava S, Gumbo T (2011) In vitro and in vivo modeling of tuberculosis drugs and its impact on optimization of doses and regimens. Curr Pharm Des 17(27):2881–2888
de Steenwinkel JE, Aarnoutse RE, de Knegt GJ, Ten Kate MT, Teulen M, Verbrugh HA, Boeree MJ, van Soolingen D, Bakker-Woudenberg IA (2013) Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment, using a murine model. Am J Respir Crit Care Med 187(10):1127–1134
Radboud University (2013) Pharmacokinetics and pharmacodynamics of high versus standard dose rifampicin in patients with pulmonary tuberculosis (High RIF). In: http://www.ClinicalTrials.gov [Internet], Bethesda (MD): National Library of Medicine (US). Available from: http://www.clinicaltrials.gov/ct2/show/results/NCT00760149, nLM Identifier: NCT00760149
Radboud University (2014) Safety, tolerability, extended early bactericidal activity and PK of higher doses rifampicin in adults with pulmonary TB (HR1) In: http://www.ClinicalTrials.gov [Internet], Bethesda (MD): National Library of Medicine (US). Available from: http://clinicaltrials.gov/ct2/show/results/NCT00760149, nLM Identifier: NCT00760149
McCune RM, Tompsett R (1956) Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med 104(5):737–762
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26(1):1–10
Hoff DR, Ryan GJ, Driver ER, Ssemakulu CC, De Groote MA, Basaraba RJ, Lenaerts AJ (2011) Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PLoS One 6(3):e17550
Dhar N, McKinney JD (2007) Microbial phenotypic heterogeneity and antibiotic tolerance. Curr Opin Microbiol 10(1):30–38
Rocco A, Kierzek AM, McFadden J (2013) Slow protein fluctuations explain the emergence of growth phenotypes and persistence in clonal bacterial populations. PLoS One 8(1):e54272
Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, McKinney JD (2013) Dynamic persistence of antibiotic-stressed mycobacteria. Science 339(6115):91–95
Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL (2007) Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51(11):3781–3788
Geli P, Andersson M, Svensson A, Andersson DI (2009) A multi-type branching model with varying environment for bacterial dynamics with postantibiotic effect. J Theor Biol 256(1):58–64
Geli P (2009) Modeling the mechanism of postantibiotic effect and determining implications for dosing regimens. J Math Biol 59(5):717–728
Lenaerts AJ, Gruppo V, Brooks JV, Orme IM (2003) Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob Agents Chemother 47(2):783–785
Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I (2005) Ipr1 gene mediates innate immunity to tuberculosis. Nature 434(7034):767–772
Flynn JL (2006) Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8(4):1179–1188
Acknowledgments
The authors wish to thank Scott Irwin and Mary Ann De Groote (Colorado State University (CSU)), Joanne Turner (The Ohio State University), and Radha Shandil (formerly AstraZeneca, Bangalore, India) for helpful discussions. The authors also thank Brad Reisfeld and Raymond Yang (CSU) for a careful review and editing of an earlier version of this manuscript. This work was supported by National Institutes of Health Grant Number K25AI089945.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
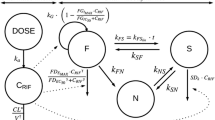
The PK/PD model used in the present work is described by Eqs. (3–23) and the corresponding compartmental structure shown in Fig. 5. The equations are those from Friedman et al. [24] and Lyons et al. [23] together with the modifications described in the "Materials and methods" section.
PK/PD model structure. a Intracellular (In) and extracellular (Ex) bacterial populations and killing kinetics parameters. \(B_{E}\): extracellular bacteria, \(B_{A}\): bacteria residing in activated macrophages, \(B_{I}\): bacteria residing in infected macrophages, \(k_{max}\): maximum kill rate, \(EC_{50}\): half-maximum effect drug concentration, and \(\gamma\): Hill coefficient. b Lung compartment with free rifampin drug concentration (\(f_{LU}C_{LU}\)) together with cell and cytokine populations (adapted from Friedman et al. [24]). \(M_{R}, M_{A}, \text {and}\; M_{I}\): resting, activated, and infected macrophages. \(I_{10}, I_{12}, I_{2}, \text {and}\; I_{\gamma }\): IL-10, IL-12, IL-2, and IFN-\(\gamma\). \(T_{4}\) and \(T_{8}\): CD4\(^{+}\) and CD8\(^{+}\) T cells. c Rifampin PBPK model compartments and blood flow (adapted from Lyons et al. [23])
Pharmacodynamics: TB model
The TB model and drug-bacteria interactions are described by Eqs. (3–13). The variable names, descriptions, and initial conditions used in the the present work are shown in Table 4. The parameter values that were not updated in the Bayesian calibration are shown in Table 5, while the updated parameters are the posterior mean values shown in Table 2.
The functions \(\lambda _{i}(t), \; i = \{x,y,z,u\}\) were implemented here as \(\lambda _{i}(t) = \lambda _{i} \cdot \theta (t - t_{delay})\), where \(t_{delay} = 14\) days, and \(\theta (x) = 1 (0)\) for \(x\ge 0(x<0)\) is a step function.
Pharmacokinetics: PBPK model
The PBPK model is described by Eqs. (14–23) with initial conditions all set to zero. The subscripts, T, on the tissue volumes, \(V_{T}\), drug concentrations, \(C_{T}\), blood flow rates, \(Q_{T}\), and tissue/blood partition coefficients, \(P_{T}\), denote abbreviations for the model compartments as; V: venous blood, LU: lung, A: arterial blood, BR: brain, F: fat, SK: skin, K: kidney, S: spleen, G: gut, GL: gut lumen, L: liver, LA: hepatic artery, CR: carcass. Fractional tissue volumes, \(V_{TC}\), and blood flow rates, \(Q_{TC}\), were scaled to total values as \(V_{T} = V_{TC}\cdot BW\) and \(Q_{T} = Q_{CC}\cdot BW^{0.75}\). BW is body weight, and \(Q_{CC}\) is an allometric coefficient for cardiac output. Fractional clearance was also scaled as \(CL = CL_{C} \cdot BW^{0.75}\). Drug concentration in plasma was determined from concentration in venous blood as \(C_{plasma} = C_{V}/BP\). The parameter values that were not updated in the Bayesian calibration are shown in Tables 6 and 7, while the updated parameters are given in Table 2.
The summation in Eq. (14) is over all tissues draining into the venous blood compartment; for liver, \(Q_{L} = \left( Q_{LA}+Q_{S}+Q_{G} \right)\). The symbols \(A_{OD}\) and \(A_{GL}\) in Eqs. (21–23) are the amounts of drug input to the gut and in the gut lumen. The delta function in Eq. (23) describes pulsed oral bolus dosing (D = dose), with times of administration, \(t_{n}, \; n = 0, \ldots , n_{max}-1\), with \(n_{max}\) the maximum number of doses in the treatment interval.
Rights and permissions
About this article
Cite this article
Lyons, M.A., Lenaerts, A.J. Computational pharmacokinetics/pharmacodynamics of rifampin in a mouse tuberculosis infection model. J Pharmacokinet Pharmacodyn 42, 375–389 (2015). https://doi.org/10.1007/s10928-015-9419-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9419-z