Abstract
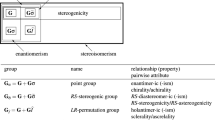
The partial-cycle-index (PCI) method of the unit-subduced-cycle-index approach (Fujita in Symmetry and combinatorial enumeration of chemistry. Springer, Berlin, 1991) is extended to meet the stereoisogram approach (Fujita in J Org Chem 69:3158–3165, 2004, Tetrahedron 60:11629–11638, 2004). Then, the PCI method is applied to the symmetry-itemized enumeration of quadruplets of stereoisograms, where an allene skeleton is considered to belong to the RS-stereoisomeric group \(\mathbf{D}_{2d{\widetilde{\sigma }}\widehat{I}}\) derived from the point group \(\mathbf{D}_{2d}\). The resulting numbers of quadruplets are itemized in terms of subgroups of \(\mathbf{D}_{2d{\widetilde{\sigma }}\widehat{I}}\), which are further categorized into five types (types I–V). The term ‘absolute configuration’, which has been correlated solely to chirality in the conventional stereochemistry, is extended to have three aspects (i.e., a chiral aspect, an RS-stereogenic aspect, and a scleral aspect) according to the three attributes of a stereoisogram (i.e., chirality, RS-stereogenicity, and sclerality). Thereby, the RS-stereodescriptors of the Cahn–Ingold–Prelog system are clarified to specify the RS-stereogenic aspect, not to specify the chiral aspect. They are assigned to a pair of RS-diastereomers contained in a type-I, type-III, or type-V stereoisogram, but not to a pair of enantiomers. To judge whether or not the RS-stereodescriptors (assigned originally on the basis of the RS-stereogenic aspect of an absolute configuration) are permitted to be applied to the chiral aspect, the concept of chirality faithfulness (Fujita in J Comput Aided Chem 10:16–29, 2009) is used after redefined by proposing odd and even priority permutations.






















Similar content being viewed by others
References
R.S. Cahn, C.K. Ingold, V. Prelog, Angew. Chem. Int. Ed. Eng. 5, 385–415 (1966)
V. Prelog, G. Helmchen, Angew. Chem. Int. Ed. Eng. 21, 567–583 (1982)
G. Helmchen, in Stereoselective Synthesis, 4th edn. ed. by G. Helmchen, R.W. Hoffmann, J. Mulzer, E. Schaumann, Georg Thieme, Stuttgart, NY (1996) Vol. 1 of Methods of Organic Chemistry (Houben-Weyl). Workbench Edition E21, pp. 1–74
S. Fujita, J. Org. Chem. 69, 3158–3165 (2004)
S. Fujita, J. Math. Chem. 35, 265–287 (2004)
S. Fujita, Tetrahedron 60, 11629–11638 (2004)
S. Fujita, MATCH Commun. Math. Comput. Chem. 54, 39–52 (2005)
S. Fujita, J. Comput. Aided Chem. 10, 16–29 (2009)
S. Fujita, Symmetry and Combinatorial Enumeration in Chemistry (Springer, Berlin, 1991)
S. Fujita, Diagrammatical Approach to Molecular Symmetry and Enumeration of Stereoisomers (University of Kragujevac, Faculty of Science, Kragujevac, 2007)
S. Fujita, Combinatorial Enumeration of Graphs, Three-Dimensional Structures, and Chemical Compounds (University of Kragujevac, Faculty of Science, Kragujevac, 2013)
S. Fujita, MATCH Commun. Math. Comput. Chem. 61, 71–115 (2009)
S. Fujita, J. Math. Chem. 33, 113–143 (2003)
S. Fujita, Memoirs of the faculty of engineering and design. Kyoto Inst. Technol. 53, 19–38 (2005)
S. Fujita, Helv. Chim. Acta 85, 2440–2457 (2002)
S. Fujita, Tetrahedron 47, 31–46 (1991)
S. Fujita, J. Math. Chem. doi:10.1007/s10910-013-0276-y (2013)
S. Fujita, J. Math. Chem. doi:10.1007/s10910-013-0277-x (2013)
S. Fujita, MATCH Commun. Math. Comput. Chem. 52, 3–18 (2004)
IUPAC Organic Chemistry Division, Pure Appl. Chem. 68, 2193–2222 (1996)
IUPAC Chemical Nomenclature and Structure Representation Division, Provisional Recommendations. Nomenclature of Organic Chemistry (2004). http://www.iupac.org/reports/provisional/abstract04/favre_310305.html
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, S. Symmetry-itemized enumeration of RS-stereoisomers of allenes: II—the partial-cycle-index method of the USCI approach combined with the stereoisogram approach. J Math Chem 52, 1751–1793 (2014). https://doi.org/10.1007/s10910-014-0345-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-014-0345-x