Abstract
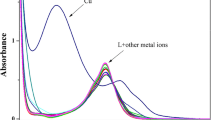
A new fluorescent sensor, 4-allylamine-N-(N-salicylidene)-1,8-naphthalimide (1), anchoring a naphthalimide moiety as fluorophore and a Schiff base group as receptor, was synthesized and characterized. The photophysical properties of sensor 1 were conducted in organic solvents of different polarities. Our study revealed that, depending on the solvent polarity, the fluorescence quantum yields varied from 0.59 to 0.89. The fluorescent activity of the sensor was monitored and the sensor was consequently applied for the detection of Cu2+ with high selectivity over various metal ions by fluorescence quenching in Tris-HCl (pH = 7.2) buffer/DMF (1:1, v/v) solution. From the binding stoichiometry, it was indicated that a 1:1 complex was formed between Cu2+ and the sensor 1. The fluorescence intensity was linear with Cu2+ in the concentration range 0.5–5 μM. Moreso, the detection limit was calculated to be 0.32 μM, which is sufficiently low for good sensitivity of Cu2+ ion. The binding mode was due to the intramolecular charge transfer (ICT) and the coordination of Cu2+ with C = N and hydroxyl oxygen groups of the sensor 1. The sensor proved effective for Cu2+ monitoring in real water samples with recovery rates of 95–112.6 % obtained.








Similar content being viewed by others
References
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Chem Rev 97:1515–1566
Gunnlaugsson T, Lee TC, Parkesh R (2003) Org Biomol Chem 1:3265–3267
Misra A, Shahid M, Srivastava P (2012) Sensors Actuators B 169:327–340
Bojinov VB, Panova IP, Simeonov DB, Georgiev NI (2010) J. Photochem. Photobiol A: Chem, 210:89–99
Li Q, Guo Y, Shao SJ (2012) Sensors Actuators B 171–172:872–877
Bargossi C, Fiorini MC, Montalti M, Prodi L, Zaccheroni N (2000) Coord Chem Rev 208:17–32
Aragay G, Pons J, Merkoci A (2011) Chem Rev 111(5):3433–3458
Quang DT, Kim JS (2010) Chem Rev 110:6280–6301
Nolan EM, Lippard SJ (2008) Chem Rev 108(9):3443–3480
Hewage HS, Anslyn EV, Am J (2009) Chem Soc 131(36):13099–13106
Kaur N, Kumar S (2011) Tetrahedron 67(48):9233–9264
Kuang GC, Allen JR, Baird MA, Nguyen BT, Zhang L, Morgan Jr TJ, Levenson CW, Davidson MW, Zhu L (2011) Inorg Chem 50(20):10493–10504
Dutta M, Das D (2012) Trac-trends Anal Chem 32:113–132
Kim HN, Ren WX, Kim JS, Yoon J (2012) Chem Soc Rev 41:3210–3244
Barba-Bon A, Costero AM, Gil S, Parra M, Soto J, Martínez-Máñez R, Sancenón F (2012) Chem Commun 48:3000–3002
Valeur B, Leray I (2000) Coord Chem Rev 205(1):3–40
Que EL, Domaille DW, Chang CJ (2008) Chem Rev 108(5):1517–1549
Cho J, Pradhan T, Lee YM, Kim JS, Kim S (2014) Dalton Trans 43:16178–16182
You GR, Park GJ, Lee JJ, Kim C (2015) Dalton Trans 44:9120–9129
Tapiero H, Townsend DM, Tew KD (2003) Biomed Pharmacother 57(9):386–398
Barnham KJ, Masters CL, Bush AI (2004) Nat Rev Drug Discov 3:205–214
Mare S, Penugonda S, Robinson SM, Dohgu S, Banks WA, Ercal N (2007) Peptides 28(7):1424–1432
Kim BE, Nevitt T, Thiele DJ (2008) Nat Chem Biol 4:176–185
Lee JC, Gray HB, Winkler JR (2008) J Am Chem Soc 130(22):6898–6899
Mokhir A, Kiel A, Herten D-P, Kraemer R (2005) Inorg Chem 44(16):5661–5666
Liu J, Lu Y, Am J (2007) Chem Soc 129(32):9838–9839
Løvstad RA (2004) Biometals 17(2):111–113
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Chem Rev 112:1910–1956
Fabbrizzi L, Licchelli M, Pallavicini P, Sacchi D, Taglietti A (1996) Analyst 121:1763–1768
Kramer R (1998) Angew Chem Int Ed 37:772–773
Nakaya KI, Funabiki R, Muramatsu H, Shabata K, Matsui M (1999) Dyes Pigments 43:235–239
Kawai K, Kawabata K, Tojo S, Majima T (2002) Bioorg Med Chem Lett 12:2363–2366
Ramachandram B, Saroja G, Sankaran NB, Samanta A (2000) J Phys Chem B 104:11824–11832
Das SK, Sahu PK, Kar UP, Rahaman A, Sarkar M, Soni M (2013) J Phys Chem C 117:14338–14347
Huang X, Fang Y, Li X, Xie Y, Zhu W (2011) Dyes Pigments 90:297–303
Mohan V, Nijamudheen A, Das SK, Sahu PK, Kar UP, Rahaman A, Sarkar M (2012) Chem Phys Chem 13:3882–3892
Wang J, Yang L, Hou C, Cao H (2012) Org Biomol Chem 10:6271–6274
Gunnlaugsson T, Kruger P, Jensen P, Pfeffer F, Hussey G (2003) Tetrahedron Lett 44:8909–8913
Desislava S, Evgenia VT, Ivo G (2014) J Mol Struct 1071:88–94
McGehee MD, Heeger AJ (2000) Adv Mater 12:1655
Bojinov V, Grabchev I (2001) Dyes Pigments 51:57–61
Tian H, He Y, Chang CP (2000) J Mater Chem 10:2049–2055
Yang S, Meng F, Tian H, Chen K (2002) Eur Polym J 38:911–919
Duke RM, Veale EB, Pfeffer FM, Kruger PE, Gunnlaugsson T (2010) Chem Soc Rev 39:3936–3953
Wang B, Anslyn EV (2011) Chemosensors: Principles, Strategies and Applications,. John Wiley & Sons, New York, pp. 229–252
Qian X, Xiao Y, Xu Y, Guo X, Qian J, Zhu W (2010) Chem Commun 46:6418–6436
M. F. Brana and A. Ramos, Curr. Med. Chem. Anti-cancer Agents., 1, 237–255 (2001)
Beezer A, Miles R, Shaw E, Willis P (1979) Experientia 35:795–796
Alexiou MS, Tychopoulos V, Ghorbanian S, Tyman JHP, Brown RG, Brittain PI (1990) J Chem Soc Perkin Trans 2:837–842
Chen ZJ, Wang LM, Zou G, Cao XM, Wu Y, Hu PJ (2013) Spectrochim Acta A 114:323–329
de Silva A, McCaughan B, McKinney B, Querol M (2003) Dalton Trans 10:1902–1913
Callan JF, de Silva AP, Magri DC (2005) Tetrahedron 61:8551–8588
T. Gunnlaugsson, C. McCoy, R. Morrow, C. Phelan and F. Stomeo, ARKIVOC VII, 216–28 (2003).
Ramachandram B (2005) J Fluoresc 15:71–83
Georgiev NI, Bojinov VB (2012) J Lumin 132:2235–2241
Sali S, Guittonneau S, Grabchev I (2006) Polym Adv Technol 17:180–185
Grabchev I, Chovelon JM (2008) Dyes Pigments 77:1–6
Poteau X, Brown AI, Brown RG, Holmes C, Matthew D (2000) Dyes Pigments 47:91–105
Qian J, Xu Y, Qian X, Wang J (2008) J Photochem Photobiol A: Chem 200:402–409
Han ZX, Zhang XB, Li Z, Gong YJ, Wu XY, Jin Z, He CM, Jian LX, Zhang J, Shen G-L, Yu R-Q (2010) Anal Chem 82(8):3108–3113
Job P (1928) Ann Chim 9:113–203
Benesi HA, Hildebrand JH, Am J (1949) Chem Soc 71:2703–2707
K. A. Connors, Binding Constants, John Wiley & Sons, New York, 339–343 (1987)
Valeur B (2000) Molecular Fluorescence. Wiley-VCH, Weinheim, pp. 339–346
Joshi BP, Park J, Lee WI, Lee KH (2009) Talanta 78:903–909
Jung HS, Kwon PS, Lee JW, Kim JII, Hong CS, Kim JW, Yan S, Lee JY, Lee JH, Joo T, Kim JS (2009) J Am Chem Soc 131(5):2008–2012
Bhardwaj VK, Pannu APS, Singh N, Hundal MS, Hundal G (2008) Tetrahedron 64:5384–5391
Pascu SI, Balazs G, Green JC, Green MLH, Vei IC, Warren JE, Windsor C (2010) Inorg Chim Acta 363:1157–1172
Acknowledgment
The authors appreciate the financial supports from the National Natural Science Foundation of China (Grant No. 21367017), Natural Science Foundation of Gansu Province (Grant No. 1212RJZA037) and Graduate Student Innovation Projects of Lanzhou Jiaotong University, which resulted in this article. Likewise, the first author thanks the Chinese Scholarship Council for awarding him a scholarship opportunity (CSC No. 2014BSZ528) to conduct his Master’s program in China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aderinto, S.O., Xu, Y., Peng, H. et al. A highly Selective Fluorescent Sensor for Monitoring Cu2+ Ion: Synthesis, Characterization and Photophysical Properties. J Fluoresc 27, 79–87 (2017). https://doi.org/10.1007/s10895-016-1936-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1936-7