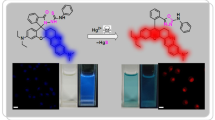
This article presents the design and synthesis of a new variant of the 4-amino-1,8-naphthalimide fluorescent probe for monitoring the divalent copper ion, Cu2+. The probe BRST, functionalized with two propionic carboxylic acid groups in its receptor moiety, demonstrated high selectivity and sensitivity for the detection of Cu2+ in competitive media of various metal ions in DMSO/HEPES buffer solution (1:1; pH 7.4), with a fluorescence quenching of 75.41%. Besides its good linearity with Cu2+, the probe yielded commendable values of the association constant and the detection limit, 5.54 × 105 M–1 and 4.33 × 10–9 M, respectively. The quenching of the fluorescence intensity of the sensor in the presence of Cu2+ ions is attributable to the strong paramagnetic nature of the Cu2+ ion. The fluorescence "turn off" behavior of this simple and effective probe was successfully applied for the determination of Cu2+ levels in untreated water samples, with excellent recovery rates of 98–105.8%.
Similar content being viewed by others
References
N. Kaur and S. Kumar, Tetrahedron, 67, 9233–9264 (2011).
A. Caballero, R. Martínez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tárraga, P. Molina, and J. Veciana, J. Am. Chem. Soc., 127, 15666–15667 (2005).
V. B. Bojinov, N. I. Georgiev, and P. S. Nikolov, J. Photochem. Photobiol. A, 193, 129–138 (2008).
W. K. Dong, S. F. Akogun, Y. Zhang, Y. X. Sun, and X. Y. Dong, Sensor. Actuat. B: Chem., 238, 723–734 (2017).
J. H. Hu, Y. Sun, J. Qi, Q. Li, and T. B. Wei, Spectrochim. Acta A, 175, 125–133 (2017).
F. Wang, Y. L. Xu, S. O. Aderinto, H. P. Peng, H. Zhang, and H. L. Wu, J. Photochem. Photobiol. A, 332, 273–282 (2017).
W. K. Dong, X. L. Li, L. Wang, Y. Zhang, and Y. J. Ding, Sensor. Actuat. B: Chem., 229, 370–378 (2016).
Y. L. Xu, S. S. Mao, H. P. Peng, F. Wang, H. Zhang, S. O. Aderinto, and H. L. Wu, J. Lumin., 192, 56–63 (2017).
S. Thavornpradit, J. Sirirak, and N. Wanichacheva, J. Photochem. Photobiol. A, 330, 55–63 (2016).
C. Barranguet, F. P. van den Ende, M. Rutgers, A. M. Breure, M. Greijdanus, J. J. Sinke, and W. Admiraal, Environ. Toxicol. Chem., 22, 1340–1349 (2003).
J. Liu and Y. Lu, J. Am. Chem. Soc., 129, 9838–9839 (2007).
Y. Sun, J. H. Hu, J. Qi, and J. B. Li, Spectrochim. Acta A, 167, 101–105 (2016).
R. Kramer, Angew. Chem. Int. Edit., 37, 772–773 (1998).
G. L. Millhauser, Acc. Chem. Res., 37, 79–85 (2004).
E. Gaggelli, H. Kozlowski, D. Valensin, and G. Valensin, Chem. Rev., 106, 1995–2044 (2006).
W. Y. Lin, L. Yuan, W. Tan, J. B. Feng, and L. L. Long, Chem. Eur. J., 15, 1030–1035 (2009).
R. Bergonzi, L. Fabbrizzi, M. Licchelli, and C. Mangano, Coord. Chem. Rev., 170, 31–46 (1998).
Q. T. Meng, Y. Shi, C. P. Wang, H. M. Jia, X. Gao, R. Zhang, Y. F. Wang, and Z. Q. Zhang, Org. Biomol. Chem., 13, 2918–2926 (2015).
R. Zhang, X. J. Yu, Y. J. Yin, Z. Q. Ye, G. L. Wang, and J. L. Yuan, Anal. Chim. Acta, 691, 83–88 (2011).
M. H. Lim, B. A. Wong, W. H. Pitcock, Jr., D. Mokshagundam, M. H. Baik, and S. J. Lippard, J. Am. Chem. Soc., 128, 14364–14373 (2006).
E. B. Veale and J. A. Kitchen, T. Gunnlaugsson, Supramol. Chem., 25, 101–108 (2013).
Z. Xu, J. Yoon, and D. R. Spring, Chem. Commun., 46, 2563–2565 (2010).
N. Singh, N. Kaur, B. McCaughan, and J. F. Callan, Tetrahedron Lett., 51, 3385–3387 (2010).
J. Huang, Y. Xu, and X. Qian, Dalton Ttans., 1761–1766 (2009).
H. L. Wu, S. O. Aderinto, Y. L. Xu, H. Zhang, and X. Y. Fan, J. Appl. Spectrosc., 84, 25–30 (2017).
E. L. Que, D. W. Domaille, and C. J. Chang, Chem. Rev., 108, 1517–1549 (2008).
Y. L. Xu, S. O. Aderinto, H. L. Wu, H. P. Peng, H. Zhang, J. W. Zhang, and X. Y. Fan, Z. Naturforsch. B, 72, 35–41 (2017).
S. O. Aderinto, Y. L. Xu, H. P. Peng, F. Wang, H. L. Wu, and X. Y. Fan, J. Fluoresc., 27, 79–87 (2017).
Y. Gao, Y. Li, X. Yang, F. He, J. Huang, M. Jiang, Z. Zhou, and H. Chen, RSC Adv., 5, 80110–80117 (2015).
Y. Zhang, X. Guo, X. Tian, A. Liu, and L. Jia, Sensor. Actuat. B: Chem., 218, 37–41 (2015).
H. L. Wu, H. P. Peng, F. Wang, H. Zhang, C. G. Chen, J. W. Zhang, and Z. H. Yang, J. Appl. Spectrosc., 83, 931–937 (2017).
J. Wang, Y. Xiao, Z. Zhang, X. Qian, Y. Yang, and Q. Xu, J. Mater. Chem., 15, 2836–2839 (2005).
S. O. Aderinto, H. Zhang, H. L. Wu, C. Y. Chen, J. W. Zhang, H. P. Peng, Z. H. Yang, and F. Wang, Color. Technol., 133, 40–49 (2017).
H. L. Wu, C. Y. Chen, H. Zhang, H. P. Peng, F. Wang, Z. H. Yang, and J. W. Zhang, Chem. Pap., 70, 685–694 (2016).
N. I. Georgiev, V. B. Bojinov, and N. Marinova, Sensor. Actuat. B: Chem., 150, 655–666 (2010).
D. Staneva, I. Grabchev, J.-P. Soumillion, and V. Bojinov, J. Photochem. Photobiol. A, 189, 192–197 (2007).
H. P. Peng, K. S. Shen, S. S. Mao, X. K. Shi, Y. L. Xu, S. O. Aderinto, and H. L. Wu, J. Fluoresc., 27, 1191–1200 (2017).
H. L. Wu, C. P. Wang, J. W. Zhang, Y. H. Zhang, C. Y. Chen, Z. H. Yang, and X. Y. Fan, Z. Naturforsch. B, 70, 863–869 (2015).
X. Poteau, A. I. Brown, R. G. Brown, C. Holmes, and D. Matthew, Dyes Pigments, 47, 91–105 (2000).
X. Sun, G. Kim, Y. Xu, J. Yoon, and T. D. James, Chem. Plus Chem., 81, 30–34 (2016).
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, New York (1999).
O. Stern and M. Volmer, Z. Phys., 20, 183 (1919).
P. Job, Ann. Chim. Appl., 9, 113–203 (1928).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publsihed in Zhurnal Prikladnoi Spektroskopii, Vol. 85, No. 4, pp. 612–619, July–August, 2018.
Rights and permissions
About this article
Cite this article
Shen, K., Mao, S., Shi, X. et al. Development of a New 4-Amino-1,8-Naphthalimide Derivative as a Fluorescent Probe for Monitoring the Divalent Copper Ion. J Appl Spectrosc 85, 665–672 (2018). https://doi.org/10.1007/s10812-018-0702-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0702-9