Abstract
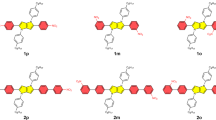
The spectral properties of a novel type of Y-shaped fluorophores consisting of an imidazole ring end-capped with two electron-donating N,N-dimethylaminophenyl groups at positions C4 and C5 and one electron-withdrawing cyano group on the imidazole moiety at position C2 were examined. The π-linker separating the 4,5-bis[4-(N,N-dimethylamino)phenyl]-1H-imidazole donor moiety and the cyano group comprises 1,4-phenylene (1), (E)-phenylethenyl (2), (E)-phenylbuta-1,3-dienyl (3), biphenyl (4), (E)-phenylethenylphenyl (5) and phenylethynylphenyl (6) conjugated paths. The absorption and fluorescence spectra were obtained in toluene, dichloromethane, acetonitrile and methanol and in polymer matrices such as polystyrene (PS), poly(methyl methacrylate) (PMMA) and poly(vinylchloride) (PVC). The most intense absorption bands of fluorophores 1–6 were observed within the range of 283 to 330 nm. Less intense but longer-wavelength absorption bands designated as charge-transfer bands were observed at approximately 380–430 nm depending on the medium. The fluorophores exhibited strong fluorescence in the visible region with a Stokes shift of approximately 4300–5800 cm−1 in non-polar toluene and polystyrene, whereas very low intensity of fluorescence was observed with a Stokes shift in the 6500–7800 cm−1 region in polar methanol and acetonitrile. The large Stokes shift indicates a large difference in the spatial arrangement of the chromophore in the absorbing and emitting states. A relatively intense fluorescence (quantum yields of 0.12–0.69) was observed only for derivative 1 in all media except methanol. The fluorophores doped in matrices yielded more intense fluorescence compared with the fluorescence in liquid media. The use of solid polymer matrices lowers the probability of forming non-emissive excited states. The fluorescence lifetimes were short (1–4 ns) for all of the fluorophores in solvents and in polymer matrices.










Similar content being viewed by others
References
Jen AK-Y, Yue Zhang (1998) Electronoptic Polymers and Applications; Materials Based on Heteroaromatic Nonlinear optical Chromophores in Photonic Polymer Systems: Fundamentals, Methods and Applications, Eds.: Wise DL, Wnek GC, Trantolo DJ, Cooper TM, Gresser JD, Macel Dekker Inc. 270 Madison Avenue, New York, NY 10016–0602, Ch. 21 pp 847
Special issue on Organic Electronics and Optoelectronics, (2007), (Eds. S. R. Forrest, M. E. Thompson). Chem Rev 107:923–1386
Special issue on Materials for electronics, (2010), (Eds. R. D. Miller, E. A. Chandross), Chem Rev 110: 1–574
Special issue on Organic Photovoltaics, (2009), (Eds.: J. L. Brédas, J. R. Durrant), Acc Chem Res 42: 1689–1857
Grabowski ZM (1993) Electron transfer in flexible molecules and molecular ions. Pure Appl Chem 65:1751–1756. doi:10.1351/pac199365081751
Safarzadeh-Amiri A (1986) A time resolved fluorescence study of dynamic stokes shift of trans-4-dimethylamino-4′-cyanostilbene. Chem Phys Lett 125:272–278. doi:10.1016/0009-2614(86)87063-4
Abdel-Mottaleb MSA, Loufty RO, Laouyade R (1989) Non-radiative deactivation channels of molecular rotors. J Photochem Photobiol A 48:87–93. doi:10.1016/1010-6030(89)87093-5
Mqadmi S, Pollet A (1990) Non-radiative deactivation of p-(N, N-dialkylamino)- benzylidenemalonitriles. J Photochem Photobiol A 53:275–281. doi:10.1016/1010-6030(90)87131-T
Paczkowski J, Neckers DC (1991) The nature of the ground and excited states of substituted (N, N-dialkylamino)cinnamated and banzalmalonate. J Photochem Photobiol A 62:173–181. doi:10.1016/1010-6030(91)87018-Q
Wang SL, Ho TI (2000) Substituent effects on intramolecular charge-transfer behaviour of styrylheterocycles. J Photochem Photobiol A 135:119–126. doi:10.1016/S1010-6030(00)00289-6
Yesodha SK, Pillai ChKS, Tsutsumi N (2004) Stable polymeric materials for nonlinear optics: a review based on azobenzene systems. Prog Polym Sci 29:45–74. doi:10.1016/j.progpolymsci.2003.07.002
Bureš F, Schweizer WB, May JC, Boudon C, Gisselbrecht J-P, Gross M, Biaggio I, Diederich F (2007) Property tuning in charge transfer chromophores by systematic modulation of the spacer between donor and acceptor. Chem Eur J 13:5378–5387. doi:10.1002/chem.200601735
Wu YL, Bureš F, Jarowski PD, Schweizer WB, Boudon C, Gisselbrecht J-P, Diederich F (2010) Proaromacity: Organic charge–transfer chromophores with Smaal HOMO-LUMO gaps. Chem Eur J 16:9592–9605. doi:10.1002/chem.201001051
Bureš F, Schweizer WB, May JC, Boudon C, Gisselbrecht J-P, Gross M, Diederich F (2008) New push-pull chromophores featuring TCAQ (11,11,12,12-tetracyano-9,10-anthraquinodimethanes) and other dicyanovinyl acceptors. Eur J Org Chem 994–1004. doi:10.1002/ejoc.200700970
Bureš F, Pytela O, Diederich F (2009) Solvent effects on electronic absorption spectra of donor substituted 11,11,12,12-tetracyano-9,10-anthraquinodimethanes (TCAQs). J Phys Org Chem 22:155–162. doi:10.1002/poc.1443
Bureš F, Pytela O, Kivala M, Diederich F (2011) Solvatochromism as an efficient tool to study N, N-Dimethylamino- and Cyano-substituted π-conjugated molecules with an intramolecular charge-transfer absorption. J Phys Org Chem 24:274–281. doi:10.1002/poc.1744
Kulhánek J, Bureš F, Pytela O, Mikysek T, Ludvík J, Růžička A (2010) Push - pull molecules with a systematically extended π-conjugated system featuring 4,5-dicyanoimidazole. Dyes Pigm 85:57–65. doi:10.1016/j.dyepig.2009.10.004
Bureš F, Kulhánek J, Mikysek T, Ludwig J, Lokaj J (2010) Branched charge transfer chromophores featuring a 4,5-dicyanoimidazole unit. Tetrahedron Lett 51:2055–2058. doi:10.1016/j.tetlet.2010.02.067
Kulhánek J, Bureš F, Wojciechowski A, Makowska-Janusik M, Gondek E, Kityk VI (2010) Optical operation by chromophores featuring 4,5-dicyanoimidazole embedded within poly(methyl methacrylate) matrices. J Phys Chem A 114:9440–9446. doi:10.1021/jp1047634
Nepraš M, Almonasy N, Bureš F, Kulhánek J, Dvořák M, Michl M (2011) Fluorescence and photophysical properties of d-π-a systems featuring a 4,5-dicyanoimidazole unit. Dyes Pigm 91:466–473. doi:10.1016/j.dyepig.2011.03.025
Kulhánek J, Bureš F, Mikysek T, Ludvík J, Pytela O (2011) Imidazole as a central π-linkage in Y-shaped push - pull chromophores. Dyes Pigm 90:48–55. doi:10.1016/j.dyepig.2010.11.004
Kulhánek J, Bureš F, Pytela O, Mikysek T, Ludvík J (2011) Imidazole as a donor/acceptor unit in charge-transfer chromophores with extended π-linkers. Chem Asian J 6:1604–1612. doi:10.1002/asia.201100097
Plaquet A, Champagne B, Kulhánek J, Bureš F, Bogdan E, Castet F, Ducasse L, Rodriguez V (2011) Effects of the nature and length of the π-conjugated bridge on the second-order nonlinear optical responses of push-pull molecules including 4,5-dicyanoimidazole and its protonated forms. Chem Phys Chem 12:3245–3252. doi:10.1002/cphc.201100299
Jaing G, Michinobu T, Yuan W, Feng M, Wen Y, Du S, Gao H, Jiang L, Song Y, Diederich F, Zhu D (2005) Crystalline thin fims of s donor-substituted cyanoethene for nanoscale data recording through intramolecular charge-transfer interaction. Adv Mater 17:2170–2173. doi:10.1002/adma.200500559
Haldi A, Kimyonok A, Domercq B, Hayden LE, Jones SC, Marder SR, Weck M, Kippelen B (2008) Optimization of orange-emitting electrophosphorescent copolymers for organic light emitting diodes. Adv Funct Mater 18:3056–3062. doi:10.1002/adfm.200800446
Innocenzi P, Lebeau B (2005) Organic–inorganic hybrid materials for non-linear optics. J Mater Chem 15:3821–3831. doi:10.1039/b506028a
Cho MJ, Choi DH, Sullivan PA, Akelaitis APJ, Dalton LR (2008) Recent progress in second non-linear polymer and dendrimers. Prog Polym Sci 33:1013–1058. doi:10.1002/adma.200500559
Ma H, Lui S, Luo J, Sures S, Lui I, Kang SH, Haller M, Sassa T, Dalton LR, Jen AK-Y (2002) Highly efficient and thermally stable electro-optical polymers and dendrimers. Adv Funct Mater 12:565–574. doi:10.1002/1616-3028(20020916
Danko M, Hrdlovič P, Kulhánek J, Bureš F (2011) Push-pull fluorophores based on imidazole-4,5-dicarbonitrile: a comparison of spectral properties in solution and in polymer matrices. J Fluores 21:1779–1787. doi:10.1016/j.dyepig.2011.07.011
Birks JB (1968) Photophysics of Aromatic Molecules. Willey-Interscience a Division of John Wiley and Sons Ltd, New York, London, Toronto, Sidney, Ch 4 pp 121–127
Kawski A, Kubicki A, Kuklinski B, Gryczynski I (1993) Unusual absorption and fluorescence properties of 1,6-diphenyl-1,3,5-hexatriene in poly(vinyl alcohol) film. J Photochem Photobiol A 71:161–167. doi:10.1016/1010-6030(93)85068-J
Chorvat D Jr, Chorvatova A (2006) Spectrally resolved time-correlated single photon counting: a novel approach for characterization of endogenous fluorescence in isolated cardiac myocytes. Eur Biophys J 36:73–83. doi:10.1007/s00249-006-0104-4
Adamson AW, Demas JN (1971) Evaluation of photoluminescence lifetimes. J Phys Chem 57:2463
Demas JN (1973) Excited state lifetime measurements, Appendix E. Academic Press, New York, p 245
Enderlein J, Erdmann R (1997) Fast fitting of multi-exponential decay curves. Opt Commun 134:371–378, (http://www.joerg-enderlein.de/fluo/fluo.html
Morimoto A, Yatsuhashi T, Shimada T, Biczók L, Tryk DA, Inoue H (2001) Radiationless deactivation of intramolecular charge transfer excited state through hydrogen bonding: effect of molecular structure and hard-soft anionic character in the excited state. J Phys Chem A 105:10488–10496. doi:10.1021/jp0117213
Danko M, Kosa C, Andics A, Vegh D, Hrdlovic P (2012) Spectral properties of chalcone containing triphenylamino structural unit in solution and in polymer matrices. Dyes Pigm 92:1257–1265. doi:10.1016/j.dyepig.2011.07.011
Bangar Raju B, Varadarajan TS (1995) Spectrosopic studies of 7-dimethylamino-3-styryl coumarins. J Photochem Photobiol A 85:263–267. doi:10.1016/1010-6030(94)03905-A
Hrobarik P, Sigmundova I, Zahradnik P, Kasak P, Arion V, Franz E, Clays K (2010) Molecular engineering of benzothiazolium salt with large quadratic hyperpolarizabilities: can auxiliary electron withdrawing groups enhance nonlinear optical response? J Phys Chem C 114:22289–22302. doi:10.1021/jp108623d
Acknowledgement
M. Danko and P. Hrdlovič thank grant agency VEGA for support of project 2/0074/10. This publication is the result of the project implementation: Centre for materials, layers and systems for applications and chemical processes under extreme conditions Stage II supported by the Research & Development Operational Programme funded by the ERDF. J. Kulhánek and F. Bureš thank the Ministry of Education, Youth and Sport of the Czech Republic MSM 0021627501.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danko, M., Bureš, F., Kulhánek, J. et al. Spectral Properties of Y-Shaped Donor-Acceptor Push-Pull Imidazole-based Fluorophores: Comparison between Solution and Polymer Matrices. J Fluoresc 22, 1165–1176 (2012). https://doi.org/10.1007/s10895-012-1056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-012-1056-y