Abstract
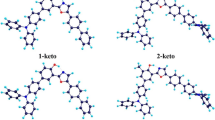
The excited-state intramolecular proton transfer (ESIPT) reaction of 3-hydroxyflavone (3-HF) in methylcyclohexane solvent has been investigated by using the DFT and TD-DFT methods. The geometric structure, IR vibrational spectra, frontier molecular orbitals, natural bond orbital, and potential energy curves in the ground state (S0) and first excited state (S1) are analyzed to reveal the mechanism of proton transfer. The results demonstrate that there are enol- and keto- two isomers for 3-HF in the S1, which is accorded with the experimental double fluorescence bands. The 3-HF-enol can be isomerized into 3-HF-keto via ESIPT. The mechanism of proton transfer is attributed to the strengthening of hydrogen bond originated from intramolecular charge transfer. The potential energy curves in the S0 and S1 states also illuminate the tautomerism mechanism between 3-HF-enol and 3-HF-keto, and the ground-state 3-HF-keto might not exist long and is isomerized mostly into the 3-HF-enol due to its high energy or instability. This is the reason that only one absorption peak is observed for 3-HF in experiment.





Similar content being viewed by others
References
C. Fang, R. R. Frontiera, R. Tran, and R. A. Mathies (2009). Nature 462, 200–205.
M. Buffa, S. Cartruan, A. Quaranta, G. Maggioni, and G. Della Mea (2012). Opt. Mater. 34, 1219–1224.
H. Li, Y. Shi, H. Yin, Y. Wang, L. Cong, M. X. Jin, and D. J. Ding (2015). Spectrochim. Acta Part A 141, 211–215.
N. Kungwan, F. Plasser, A. J. A. Aquino, M. Barbatti, P. Wolschann, and H. Lischka (2012). Phys. Chem. Chem. Phys. 14, 9016–9025.
S. C. Lan and Y. H. Liu (2015). Spectrochim. Acta Part A 139, 49–53.
P. Kukura, D. W. Mccamant, and R. A. Mathies (2007). Annu. Rev. Phys. Chem. 58, 461–488.
S. R. Meech (2009). Chem. Soc. Rev. 38, 2922–2934.
T. J. Martinez (2006). Acc. Chem. Res. 39, 119–126.
S. Hayashi, E. Taikhorshid, and K. Schulten (2009). Biophys. J. 96, 403–416.
T. Tahara, S. Takeuchi, and K. Ishii (2006). J. Chin. Chem. Soc. 53, 181–189.
T. Kobayashi, T. Saito, and H. Ohtani (2001). Nature 414, 531–534.
S. Chai, G. J. Zhao, P. Song, S. Q. Yang, J. Y. Liu, and K. L. Han (2009). Phys. Chem. Chem. Phys. 11, 4385–4390.
G. J. Zhao and K. L. Han (2008). J. Comput. Chem. 29, 2010–2017.
G. J. Zhao and K. L. Han (2007). J. Chem. Phys. 127, 024306–024312.
G. J. Zhao and K. L. Han (2007). J. Phys. Chem. A 111, 2469–2474.
G. J. Zhao, J. Y. Liu, L. C. Zhou, and K. L. Han (2007). J. Phys. Chem. B 111, 8940–8945.
G. J. Zhao and K. L. Han (2008). ChemPhysChem 9, 1842–1846.
Y. M. Dai, J. F. Zhao, Y. L. Cui, Q. Y. Wang, P. Song, F. C. Ma, and Y. Y. Zhao (2015). Spectrochim. Acta Part A 144, 76–80.
Y. L. Frolov, Y. M. Sapozhnikov, S. S. Barer, N. N. Pogodaeva, and N. A. Tyukavkina (1974). Science 23, 2279–2281.
G. J. Woolfe and P. J. Thistlethwaite (1981). J. Am. Chem. Soc. 103, 6916–6923.
M. Itoh, K. Tokumura, Y. Tanimoto, Y. Okada, H. Takeuchi, K. Obi, and I. Tanaka (1982). J. Am. Chem. Soc. 104, 4146–4150.
A. J. G. Strandjord, S. H. Courtney, D. M. Friedrich, and P. F. Barbara (1983). J. Phys. Chem. 87, 1125–1133.
D. Mcmorrow and M. Kasha (1983). J. Am. Chem. Soc. 105, 5133–5134.
A. J. G. Strandjord, D. E. Smith, and P. F. Barbara (1985). J. Phys. Chem. 89, 2362–2366.
S. M. Ormson, R. G. Brown, P. Matousek, and M. Towrie (2001). J. Phys. Chem. A 105, 3709–3718.
D. Yang, Y. Yang, and Y. Liu (2013). Commun. Comput. Chem. 1, 205–215.
M. T. Sun and H. X. Xu (2012). Small 8, 2777–2786.
J. F. Zhao, P. Song, Y. L. Cui, X. M. Liu, S. W. Sun, S. Y. Hou, and F. C. Ma (2014). Spectrochim. Acta Part A 131, 282–287.
B. K. Paul and N. Guchhait (2011). J. Lumin. 131, 1918–1926.
G. J. Zhao and K. L. Han (2008). Biophys. J. 94, 38–46.
X. H. Zhao and M. D. Chen (2010). J. Phys. Chem. A 114, 7786–7790.
K. C. Tang, C. L. Chen, H. H. Chuang, J. L. Chen, Y. J. Chen, Y. C. Lin, J. Y. Shen, W. P. Hu, and P. T. Chou (2011). J. Phys. Chem. Lett. 2, 3063–3068.
Y. Nagai, K. Saita, K. Sakata, S. Nanbu, M. Sekine, M. Nakata, and H. Sekiya (2010). J. Phys. Chem. A 114, 5041–5048.
J. F. Zhao, P. Song, and F. C. Ma (2014). Commun. Comput. Chem. 2, 117–130.
K. Ando, S. Hayashi, and S. Kato (2011). Phys. Chem. Chem. Phys. 13, 11118–11127.
M. Zhang, W. Mi, and C. Hao (2013). Commun. Comput. Chem. 1, 269–278.
B. K. Paul, A. Ganguly, and N. Guchhait (2014). Spectrochim. Acta Part A. 131, 72–81.
Y. Liu and S. C. Lan (2013). Commun. Comput. Chem. 1, 1–7.
M. T. Sun, Y. H. Chen, P. Song, and F. C. Ma (2005). Chem. Phys. Lett. 413, 110–117.
Y. Liu and S. C. Lan (2013). Commun. Comput. Chem. 1, 235–243.
P. Song, Y. Z. Li, F. C. Ma, T. Pullerits, and M. T. Sun (2013). J. Phys. Chem. C 117, 15879–15889.
F. Furche and R. Ahlrichs (2002). J. Chem. Phys. 117, 7433–7447.
J. L. Whitten (1973). J. Chem. Phys. 58, 4496–4501.
A. Schafer, C. Huber, and R. Ahlrichs (1994). J. Chem. Phys. 100, 5829–5835.
G. J. Zhao and K. L. Han (2012). Acc. Chem. Res. 45, 404–413.
R. Wu, P. Nachtigall, and B. Brutschy (2004). Phys. Chem. Chem. Phys. 6, 515–521.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Scalmani, G. Cheeseman, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr. J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision A.02 (Gaussian, Inc., Wallingford, 2009).
A. J. G. Strandjord and P. F. Barbara (1985). J. Phys. Chem. 89, 2355–2366.
D. Mcmorrow and M. Kasha (1984). J. Phys. Chem. 88, 2235–2243.
P. K. Mandal and A. Samanta (2003). J. Phys. Chem. A 107, 6334–6339.
J. F. Zhao, J. S. Chen, Y. L. Cui, J. Wang, L. X. Xia, Y. M. Dai, P. Song, and F. C. Ma (2015). Phys. Chem. Chem. Phys. 17, 1142–1150.
L. Serrano-Andres and M. Merchan (2009). J. Photochem. Photobiol. C 10, 21–32.
Y. Saga, Y. Shibata, and H. Tamiaki (2010). J. Photochem. Photobiol. C 11, 15–24.
J. F. Zhao, H. B. Yao, J. Y. Liu, and M. R. Hoffmann (2015). J. Phys. Chem. A 119, 681–688.
A. L. Sobolewski and W. Domcke (1999). Phys. Chem. Chem. Phys. 1, 3065–3072.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (Grant No. 21203012), Liaoning Excellent Talents Programand (LJQ2013118), and the Foundation of State Key Laboratory of Explosion Science and Technology of Beijing Institute of Technology (KFJJ14-08M).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Peng, Y. Excited-State Intramolecular Proton Transfer Reaction of 3-Hydroxyflavone. J Clust Sci 26, 1983–1992 (2015). https://doi.org/10.1007/s10876-015-0893-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0893-7