Abstract
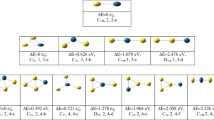
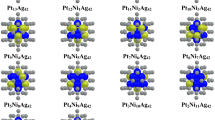
Understanding the interaction of the passivating ligands with the clusters is definitely important for the synthesization of high-quality bimetallic clusters (or “nanoalloys”) by chemical reduction method. In this work, the influence of the ligands (PH3 and PMe3) on the geometric and electronic properties of 55-atom Au–Pd nanoalloys is investigated by density functional theory calculations. It is found that the adsorption of PH3 on the Au-rich clusters can modify the morphology of the cluster and the adsoprtion strength of PH3 can be enhanced by the increase of the concentration of Pd in Au–Pd nanoalloys. Our results also suggest that increasing the number of alkyl substituents from PH3 to PMe3 can greatly enhance the adsoprtion strength of the ligand, but is of little effect on the stability of the cluster. In addition, the increase of the coverage of ligands on Au–Pd nanoalloys can slightly decrease the adsorption strength per ligand, but can significantly increase the stability of the cluster. Our results highlight a fundamental research on the effect of the ligands on the physicochemical properties of nanoalloys, which can be used to guide the synthesization of high-quality nanoalloys in the presence of passivating ligands.










Similar content being viewed by others
References
P. Taladriz-Blanco, V. Pastoriza-Santos, J. Pérez-Juste, and P. Hervés (2013). Langmuir 29, 8061.
J. F. Parker, K. A. Kacprza, O. Lopez-Acevedo, H. Häkkinen, and W. Murray (2010). J. Phys. Chem. C 114, 8276.
M. Dubecký and H. Su (2012). J. Phys. Chem. C 116, 17714.
F. Hidalgo and C. Noguez (2012). ACS Nano 7, 513.
I. V. Yudanov, A. Genest, S. Schauermann, H.-J. Freund, and N. Rösch (2012). Nano Lett. 12, 2134.
W. D. Michalak, J. M. Krier, K. Komvopoulos, and G. A. Somorjai (2012). J. Phys. Chem. C 117, 1809.
Y. Song, J. Ding, and Y. Wang (2012). J. Phys. Chem. C 116, 11343.
G. Barcaro, M. Causa, and A. Fortunelli (2007). Theor. Chem. Acc. 118, 807.
X. F. Yang, A. Q. Wang, B. T. Qiao, J. Li, J. Y. Liu, and T. Zhang (2013). Acc. Chem. Res. 46, 1740.
D. J. Cheng and W. C. Wang (2012). Nanoscale 4, 2408.
M. M. Hu, D. P. Linder, M. B. Nardelli, and A. Striolo (2013). J. Phys. Chem. C 117, 15050.
T. L. Tan, L. L. Wang, D. D. Johnson, and K. W. Bai (2012). Nano Lett. 12, 4875.
R. Ismail, R. Ferrando, and R. L. Johnston (2013). J. Phys. Chem. C 117, 293.
D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight, and G. J. Hutchings (2006). Science 311, 362.
Y. F. Han, D. Kumar, and D. W. Goodman (2005). J. Catal. 230, 353.
C. J. Baddeley, R. M. Ormerod, A. W. Stephenson, and R. M. Lambert (1995). J. Phys. Chem. 99, 5146.
C. J. Baddeley, M. Tikhov, C. Hardacre, J. R. Lomas, and R. M. Lambert (1996). J. Phys. Chem. 100, 2189.
K. Deplanche, I. P. Mikheenko, J. A. Bennett, M. Merroun, H. Mounzer, J. Wood, and L. E. Macaskie (2011). Top Catal. 54, 1110.
J. K. Edwards, B. Solsona, N. N. Edwin, A. F. Carley, A. A. Herzing, C. J. Kiely, and G. J. Hutchings (2009). Science 323, 1037.
J. K. Edwards and G. J. Hutchings (2008). Angew. Chem. Int. Ed. 47, 9192.
T. Udayabhaskararao and T. Pradeep (2013). J. Phys. Chem. Lett. 4, 1553.
M. R. Knecht, M. G. Weir, A. I. Frenkel, and R. M. Crooks (2008). Chem. Mater. 20, 1019.
B. M. Munoz-Flores, B. I. Kharisov, V. M. Jimenez-Perez, P. E. Martinez, and S. T. Lopez (2011). Ind. Eng. Chem. Res. 50, 7705.
N. J. S. Costa and L. M. Rossi (2012). Nanoscale 4, 5826.
D. Astruc, F. Lu, and J. R. Aranzaes (2005). Angew. Chem. Int. Ed. 44, 7852.
J. A. Dahl, B. L. S. Maddux, and J. E. Hutchison (2007). Chem. Rev. 107, 2228.
A. M. Doyle, S. K. Shaikhutdinov, S. D. Jackson, and H.-J. Freund (2003). Angew. Chem. Int. Ed. 42, 5240.
M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz, and R. Jin (2008). J. Am. Chem. Soc. 130, 5883.
H. Yoo, S. K. Moon, T. Hwang, Y. S. Kim, J. H. Kim, S. W. Choi, and J. H. Kim (2013). Langmuir 29, 5962.
D. Manzoor, S. Pal, and S. Krishnamurty (2013). J. Phys. Chem. C 117, 20982.
J. Kilmartin, R. Sarip, R. Grau-Crespo, D. Di Tommaso, G. Hogarth, C. Prestipino, and G. Sankar (2012). ACS Catal. 2, 957.
G. Li and R. Jin (2013). Acc. Chem. Res. 46, 1749.
M. Sankar, Q. He, M. Morad, J. Pritchard, S. J. Freakley, J. K. Edwards, S. H. Taylor, D. J. Morgan, A. F. Carley, D. W. Knight, C. J. Kiely, and G. J. Hutchings (2012). ACS Nano 6, 6600.
I. Geukens and D. E. De Vos (2013). Langmuir 29, 3170.
J. P. Wilcoxon and B. L. Abrams (2006). Chem. Soc. Rev. 35, 1162.
C. Kumara and A. Dass (2011). Nanoscale 3, 3064.
K. Naoe, C. Petit, and M. P. Pileni (2008). Langmuir 24, 2792.
J. L. Hueso, V. Sebastian, A. Mayoral, L. Uson, M. Arruebo, and J. Santamaria (2013). RSC Adv. 3, 10427.
G. Shafai, S. Y. Hong, M. Bertino, and T. S. Rahman (2009). J. Phys. Chem. C 113, 12072.
S. Knoppe, S. Malola, L. Lehtovaara, T. Burgi, and H. Hakkinen (2013). J. Phys. Chem. A 117, 10526.
O. Lopez-Acevedo, K. A. Kacprzak, J. Akola, and H. Hakkinen (2010). Nat. Chem. 2, 329.
G. Paolo, B. Stefano, B. Nicola, C. Matteo, C. Roberto, C. Carlo, C. Davide, L. C. Guido, C. Matteo, D. Ismaila, C. Andrea Dal, G. Stefano de, F. Stefano, F. Guido, G. Ralph, G. Uwe, G. Christos, K. Anton, L. Michele, M.-S. Layla, M. Nicola, M. Francesco, M. Riccardo, P. Stefano, P. Alfredo, P. Lorenzo, S. Carlo, S. Sandro, S. Gabriele, P. S. Ari, S. Alexander, U. Paolo and M. W. Renata (2009). J. Phys.: Condens. Matter. 21, 395502.
J. P. Perdew, K. Burke, and M. Ernzerhof (1996). Phys. Rev. Lett. 77, 3865.
M. Methfessel and A. T. Paxton (1989). Phys. Rev. B 40, 3616.
D. Cheng, S. Huang, and W. Wang (2006). Phys. Rev. B 74, 064117.
F. Baletto, C. Mottet, and R. Ferrando (2003). Phys. Rev. Lett. 90, 135504.
H. Zhang, T. Watanabe, M. Okumura, M. Haruta, and N. Toshima (2012). Nat. Mater. 11, 49.
G. Barcaro and A. Fortunelli (2007). New J. Phys. 9, 22.
P. S. West, R. L. Johnston, G. Barcaro, and A. Fortunelli (2010). J. Phys. Chem. C 114, 19678.
E. Apra, F. Baletto, R. Ferrando, and A. Fortunelli (2004). Phys. Rev. Lett. 93, 065502.
F. Baletto and R. Ferrando (2005). Rev. Mod. Phys. 77, 371.
F. Chen, B. C. Curley, G. Rossi, and R. L. Johnston (2007). J. Phys. Chem. C 111, 9157.
C. di Paola and F. Baletto (2011). Phys. Chem. Chem. Phys. 13, 7701.
S. Goel, K. A. Velizhanin, A. Piryatinski, S. Tretiak, and S. A. Ivanov (2010). J. Phys. Chem. Lett. 1, 927.
Acknowledgments
We would like to thank Dr. Alessandro Fortunelli for helpful comments and suggestions. This work is supported by the National Natural Science Foundation of China (21106003, 91334203), Beijing Novel Program (Z12111000250000), “Chemical Grid Project” of BUCT and Supercomputing Center of Chinese Academy of Sciences (SCCAS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Cheng, D. Effect of the Passivating Ligands on the Geometric and Electronic Properties of Au–Pd Nanoalloys. J Clust Sci 26, 799–813 (2015). https://doi.org/10.1007/s10876-014-0755-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0755-8