Abstract
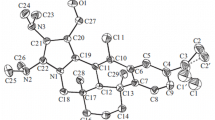
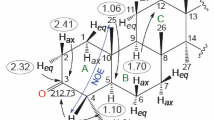
Palladium catalyzed cross coupling between 3β-acetoxy-bisnorchol-5-en-22-oic acid and phenylboronic acid produced the hereto unknown 22-phenyl-3β-acetoxy-bisnorchol-5-en-22-one which crystallizes in the monoclinic system with space group P2 1 . The presence of 17β-substituent that bears both the C21 methyl group and the introduced phenylketone moiety provides a twisted conformation on C13–C14 for the D ring with asymmetry parameters (Altona et al., Tetrahedron 24:13–32, 1968): ∆ = 711.4, τm = 47.3 (2), ∆C2(C13–C14) = 5.7 (3), ∆Cs(C13) = 14.0 (3) and ∆Cs(C14) = 21.6 (3)°. The angle 77.20 (8)° between planes of the steroid-ABCD framework and phenyl ring evidences the relative orthogonal positions of these fragments. The 1H and 13C NMR characterization of the obtained compound are described.
Graphical Abstract
The crystal structure and NMR characterization of 22-phenyl-3β-acetoxy-bisnorchol-5-en-22-one are described.




Similar content being viewed by others
References
Lednicer D (2009) Strategies for organic drug synthesis, 2nd edn. Wiley, Hoboken and references therein
Lee E, Liu YT, Solomon PH, Nakanishi K (1976) J Am Chem Soc 98:1634–1635
Lee S, LaCour TG, Fuchs PL (2009) Chem Rev 109:2275–2314
Gryszkiewicz-Wojtkielewicz A, Jastrzębska I, Morzycki JW, Romanowska DB (2003) Curr Org Chem 2003(7):1257–1277
Iglesias-Arteaga MA, Morzycki JW (2013) Cephalostatins and Ritterazines. In: Knölker HJ (ed) The Alkaloids: Chemistry and Biology, vol 72. Elsevier, Amsterdan, pp 153–279
Romero-Ávila M, de Dios-Bravo G, Méndez-Stivalet JM, Rodríguez-Sotres R, Iglesias-Arteaga MA (2007) Steroids 72:955–959
Rosado-Abón A, de Dios-Bravo G, Rodríguez-Sotres R, Iglesias-Arteaga MA (2012) Steroids 77:461–466
Rosado-Abón A, de Dios-Bravo G, Rodríguez-Sotres R, Iglesias-Arteaga MA (2013) J Steroid Biochem & Mol Biol 134:45–50
Mayorquin-Torres MC, Romero-Ávila M, Flores-Alamo M, Iglesias-Arteaga MA (2013) Steroids 78:1092–1097
CrysAlis CCD and CrysAlis R (2009) Oxford Diffraction, Abingdon
Clark RC, Reid JS (1995) Acta Crystallogr A51:887–897
Sheldrick GM (2008) Acta Crystallogr A64:112–122
Farrugia LJ (1997) Appl Crystallogr 30:565
Farrugia LJ (1999) J Appl Crystallogr 32:837–838
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Duax WL, Weeks CM, Rohrer DC (1976) Topics in Stereochemistry. Eliel E.L. Allinger N, Vol. 2. John Wiley, New York, p 271–283
Altona C, Geise HJ, Romers C (1968) Tetrahedron 24:13–32
Acknowledgements
The authors acknowledge the financial support provided by Dirección General de Asuntos del Personal Académico (Project DGAPA-IN211714) and the Faculty of Chemistry-UNAM (PAPIIT-5000-9063). We want to express our gratitude to Dr. Carlos Cobas from Mestrelab® for assistance with the MestreNova NMR processing program and to Minerva Monroy-Barreto (USAI) for recording NMR spectra. Thanks are due to CONACYT-México for the scholarship granted to M.C.M-T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayorquín-Torres, M.C., Flores-Álamo, M. & Iglesias-Arteaga, M.A. NMR Characterization and Crystal Structure of 22-Phenyl-3β-acetoxy-bisnorchol-5-en-22-one. J Chem Crystallogr 44, 501–505 (2014). https://doi.org/10.1007/s10870-014-0539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-014-0539-x