Abstract
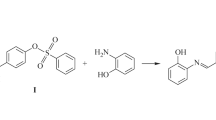
Two new derivatives of hydroxamic acid having the general formula RC(O)N(RN)OH (R = alkyl/aryl; RN = alkyl/aryl or H), have been synthesized by the condensation method in an ice-bath. The compounds, N-methyl o-iodobenzohydroxamic acid and N-methyl o-bromobenzohydroxamic acid have been isolated as crystalline solids, stable in air and soluble in organic solvents and in aqueous alcohol solution. A systematic investigation of the derivatives were carried out both in solid and in solution. They have been structurally characterized by elemental analysis, and the results were in good agreement with the values calculated for the proposed formula. These derivatives were further investigated on the basis of FT-IR, multinuclear 1H, 13C NMR spectroscopy, and Single Crystal X-ray crystallographic studies, indicating that both the compounds are structurally similar.
Graphical Abstract
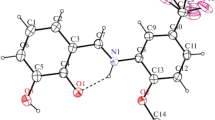
Two new compounds of hydroxamic acid have been synthesized by condensation method and were structurally characterized by elemental analysis, FT-IR, multinuclear 1H, 13C NMR spectroscopy, and Single Crystal X-ray crystallographic studies. Figure 3 Thermal ellipsoidal plot of C8 H8 Br N O2. Displacement ellipsoids are drawn at the 50 % probability level, and H atoms are shown as spheres of arbitrary radii.







Similar content being viewed by others
References
Pirrung MC, Tumey LN, McClerren AL, Raetz CRH (2003) J Am Chem Soc 125:1575
Wang W, Ryder N, Weidmann B, Patel D, Trias J, Whitea R, Yuana Z (2003) Bioorg Med Chem Lett 13:4223
Mishra H, Parrill AL, Williamsom JS (2002) Antimicrob Agents Chemother 46:2613
Muri EMF, Mishra H, Avery MA, Williamsom JS (2002) Synth Commun 33:1977
Hidalgo M, Eckhardt SG (2001) J Natl Cancer Inst 93:178
Shang XM, Wu JZ, Li QS (2006) Eur J Inorg Chem 2006:4143
Jeng AY, De Lombaert S (1997) Curr Pharm Des 3:597
Szekeres T, Fritzer-Szekeres M, Elford HL (1991) Critical 306 Rev Clin Lab Sci 34:503
El Yazal J, Pang YP (2000) J Phys Chem B 104:6499
Vippagunta SR, Dorn A, Bubendorf A, Ridley RG, Vennerstrom JL (1999) Biochem Pharmacol 58:817
Valapour M, Gou J, Schroeder JT, Keen J, Cianferoni A, Casolaro V, Georas SN (2002) J Allergy Clin Immunol 109:238
Domingo JL (1998) Reprod Toxicol 12:499
Ghosh AK (1989) Proc Indian Natl Sci Acad Part A 55:253
Thorarensen A, Douglas MR, Rohrer DC, Vosters AF, Yem AW, Marshall VD, Lynn JC, Bohanon MJ, Tomich PK, Zurenko GE, Sweeney MT, Jensen RM, Nielsen JW, Seest EP, Dolak LA (2001) Bioorg Med Chem Let 11:1355
Taira J, Chika M, Aniya Y (2002) Biochem Pharmacol 63:1019
Kalman E, Felhosi I, Karman FH, Lukovits I, Telegdi J, Palinkas G (2000) Materials Sci Technol 19(Pt.1):471
Szymanowski J (1998) Mineral Proc Extr Metall Rev 18:389
Reddy AS, Kumar MS, Reddy GR (2000) Tetrahedron Lett 41:6285
Devlin JP, Ollis WD, Thorpe JE (1975) J Chem Soc Perkin Trans 1:846
Fazary AE (2005) J Chem Eng Data 50:888
Ventura ON, Rama JB, Turi L, Dannenberg JJ (1993) J Am Chem Soc 115:5754
Bordwell FG, Fried HE, Hughes DL, Lynch TY, Satish AV, Whang YE (1990) J Org Chem 55:3330
Kakkar R, Grover R, Chadha P (2003) Org Biomol Chem 1:2200
Wu DH, Ho JJ (1998) J Phys Chem A 102:3582
Kakkar R, Grover R, Gahlot P (2006) Polyhedron 25:759
Bruker (2008) APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI
Sheldrick GM (2008) Acta Cryst A 64:112
Sheldrick GM (1997) SHELXL-97. Univ Göttingen, Germany
Qingshan L, Fa′tima M, Guedes da Silva C, Zhao J, Armando JLP (2004) J Organomet Chem 689:4584
Saxena A, Huber F, Pellerito L, Girasolo A (1986) Inorg Chim Acta 125:197
Dutta S, Deb BK, Ghosh AK (1993) Indian J Chem 32A:907
Shang X, Wu J, Pombeiro AJL, Li Qingshan (2007) Appl Organomet Chem 21:919
Kukushkin VY, Pombeiro AJL (2000) Inorg Chem 39:216
Kukushkin VY, Pakhomava TB, Kukushkin YN, Herrmann R, Wagner G, Pombeiro AJL (1998) Inorg Chem 37:6511
Zhao XJ, Zhang QF, Li DC, Dou JM, Wang DQ (2010) J Organomet Chem 695:2134
Mrinal KD, Somnath D (1995) J Organomet Chem 495:177
Bellamy LJ (1980) The infrared spectra of complex molecules. Chapman and Hall, London
Hadzi D, Pbevorsek D (1957) Fergamon Press Ltd., London. Spectrochimica Acta 9(10): 38:51
Brown DA, Coogan RA, Fitzpatrick NJ, Glass WK, Abukshima DE, Shiels L, Ahlgrén M, Smolander K, Pakkanen TT, Pakkanen TA, Perakyla M (1996) J Chem Soc Perkin Trans 2:2673
Saad E, Farina Y, Baba I, Othman H (2003) Sains Malays 32:79
Chattarjee B (1978) Coord Chem Rev 26:281
Mehrotra RC (1987) Comprehensive coordination chemistry. Pergamon Press Ltd., Oxford
Acknowledgments
This work was supported by Grant UKM-ST-06-FRGS 112-2009, UKM-GUP-NBT-08-27-112 and GUP-2012-022 and we gratefully acknowledge the School of Chemical Sciences and Food Technology, Universiti Kebangsaan Malaysia, for providing the essential laboratory facilities. We would also like to thank the Faculty Development Programme, University of Balochistan Quetta, Pakistan for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Naqeebullah, K., Farina, Y., Mun, L.K. et al. Spectral Characterization and Crystal Structures of Two Newly Synthesized Ligands of N-Methyl O-Substituted Benzohydroxamic Acids. J Chem Crystallogr 43, 622–627 (2013). https://doi.org/10.1007/s10870-013-0469-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0469-z