Abstract
We report the regioselective synthesis and X-ray structure of the pharmacologically relevant 3-[2-(7-chloro-quinolin-4-ylamino)-ethylcarbamoyl]-4-(4-methoxy-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid methyl ester (3) (triclinic, space group \( P\bar{1} \), a = 11.1775 (3), b = 13.6470 (4), c = 16.3680 (6) Å, α = 82.645 (1), β = 86.423 (1), γ = 88.415 (2)°, V = 2470.9 (1) Å3, Z = 4). Further support for the regioselectivity is provided by the X-ray structures of two intermediates, namely 4-(4-methoxy-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid methyl ester, 8 (monoclinic, space group P21/c, a = 11.7710 (2), b = 5.5290 (1), c = 22.9500 (5) Å, β = 104.342 (1)°, V = 1447.08 (5) Å3, Z = 4), and 4-(4-methoxy-phenyl)-1,3,6-trimethyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid methyl ester, 9 (monoclinic, space group P21/c, a = 16.6529 (7), b = 10.9426 (4), c = 8.2819 (3) Å, β = 91.395 (2)°, V = 1508.7 (1) Å3, Z = 4), which are also reported. The three compounds display significant differences in the conformations of their DHPM rings as well as a variety of hydrogen bonding arrangements in their crystals.
Graphical Abstract
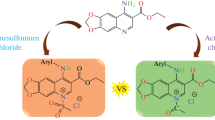
The synthetic strategy for deriving pharmacologically-relevant bifunctional compounds based on linked dihydropyrimidone and chloroquinoline moieties is reported, together with supporting X-ray structures of two synthetic intermediates and a representative target compound.








Similar content being viewed by others
References
Biginelli P (1893) Gazz Chim Ital 23:360–413
Kappe CO (2000) Eur J Med Chem 35:1043–1052
Shanmugam P, Boobalan P, Perumal PT (2007) Tetrahedron 63:12215–12219
Kappe CO (1993) Tetrahedron 49:6937–6963
Atwal KS, Rovnyak GC, Schwartz J, Moreland S, Hedberg A, Gougoutas JZ, Malley MF, Floyd DM (1990) J Med Chem 33:1510–1515
Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O’Reilly BC (1991) J Med Chem 34:806–811
Rovnyak GC, Atwal KS, Hedberg A, Kimball SD, Moreland S, Gougoutas JZ, O’Reilly BC, Schwartz J, Malley MF (1992) J Med Chem 35:3254–3263
Barrow JC, Nantermet PG, Selnick HG, Glass KL, Rittle KE, Gilbert KF, Steele TG, Homnick CF, Freidinger RM, Ransom RW, Kling P, Reiss D, Broten TP, Schorn TW, Chang RSL, O’Malley SS, Olah TV, Ellis JD, Barrish A, Kassahun K, Leppert P, Nagarathnam D, Forray C (2000) J Med Chem 43:2703–2718
Nagarathnam D, Wetzel JM, Miao SW, Marzabadi MR, Chiu G, Wong WC, Hong X, Fang J, Forray C, Branchek TA, Heydorn WE, Chang RSL, Broten T, Schorn T, Gluchowski CJ (1998) J Med Chem 41:5320–5333
Kharkar PS, Desai B, Gaveria H, Varu B, Loriya R, Naliapara Y, Shah A, Kulkarni VM (2002) J Med Chem 45:4858–4867
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Science 286:971–974
Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ (2000) J Cell Biol 150:975–988
Crevel IMTC, Alonso MC, Cross RA (2004) Curr Biol 14:R411–R412
Kyle DE, Oduola AMJ, Martin SK, Milhous WK (1990) Trans R Soc Trop Med Hyg 84:474–478
Burgess SJ, Selzer A, Xu Kelly J, Smilkstein MJ, Riscoe MK, Peyton DH (2006) J Med Chem 49:5623–5625
De D, Byers L, Krogstad DDJ (1997) J Heterocyclic Chem 34:315–320
Otwinowski Z, Minor W (1997) Methods in enzymology, Vol. 276. In: Carter CW Jr, Sweet RM (eds) Macromolecular crystallography, Part A. Academic Press, New York, pp 307–326
Sheldrick GM (1990) Acta Cryst A46:467–473
Sheldrick GM (1997) SHELXL97, Program for the refinement of crystal structures. University of Göttingen, Germany
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Li J-J, Jiang L, Wu X–P, Su W–K (2006) Acta Cryst E62:o1089–o1091
Etter MC, MacDonald JC, Bernstein J (1990) Acta Cryst B46:256–262
Acknowledgment
We thank the University of Cape Town and the NRF (Pretoria) for research support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Watermeyer, N.D., Chibale, K. & Caira, M.R. Pharmacologically Relevant Bifunctional Compounds Containing Chloroquinoline and Dihydropyrimidone Moieties: Syntheses and Crystal Structures of a Target Molecule and Selected Intermediates. J Chem Crystallogr 39, 753–760 (2009). https://doi.org/10.1007/s10870-009-9568-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9568-2