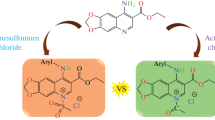
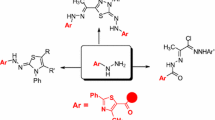
Abstract
Imidazolidine-2-thione substructure represents a pharmaceutically attractive scaffold, being included in different antimicrobial, anticancer and pesticide agents. To further evaluate the pharmaceutical potential of this chemical moiety, imidazolidine-2-thione was reacted with atypical Vilsmeier adducts, obtained by the condensation between dimethylacetamide and various acyl chlorides endowed with different electronic and steric properties. The formation of mono-acylated or di-acylated thiourea derivatives emerged to be affected by the nature of the considered acyl chloride reagent. Computational semi-empirical simulations were carried out to rationalize the relevant factor influencing the outcome of the reaction. As acylthioureas are pharmacologically relevant compounds, the chemical versatility of mono-acylated derivatives were evaluated by reacting benzoyl imidazolidin-2-thione with acyl chlorides. A small library of asymmetric di-acylthioureas was prepared and the obtained derivatives did not show any cytotoxicity on SKOV-3 and MCF-7 cancer cell lines. Additionally, in silico studies predicted good pharmacokinetics properties and promising drug-like characteristics for mono- and di-acylated thioureas. These considerations further support the value of the prepared compounds as interesting non-cytotoxic chemical scaffold useful in the medicinal chemistry field.
Graphical abstract

Similar content being viewed by others
Introduction
Acylthioureas represent a class of pharmacologically relevant compounds endowed with a number of biological properties, including antiviral [1], antibacterial [2], antituberculosis [3, 4], anticonvulsant [5], antiplatelet [6,7,8], antiarrhythmic [8], analgesic [8], antihyperlipidemic [8], anaesthetic [8], thyrostatic [9] and antiproliferative [6,7,8, 10,11,12]. Imidazolidine-2-thione derivatives were found to be active as adenosine-A2B receptor antagonists with a relevant impact for treatment and/or prophylaxis of pulmonary and cardiovascular disorders and cancers [13], as well as GPR6 inverse agonists, an orphan receptor associated with neuropsychiatric disorders [14]. Furthermore, imidazolidine-2-thione analogues have been complexed with various metals (e.g. cadmium, zinc, silver, platinum) to obtain antimicrobial or anticancer agents [15,16,17]. This scaffold has also been used for the development of effective pesticides [18] and arthropod control agents [19].
The biological activities of imidazolidine-2-thione derivatives [13,14,15,16,17,18,19] prompted us to investigate the condensation of 1 with the weak Vilsmeier reagent I, generated in situ through the reaction of N,N-dimethylformamide (DMF) and benzoyl chloride (Fig. 1) [20]. Adduct I is formed by reversible O-acylation of DMF [21,22,23,24] and proved to be a useful intermediate for the formylation of alcohols [25] and the synthesis of β-lactams [26]. Furthermore, the condensation of 1 with adduct I led to the isolation of N-methyleniminium salt Im1 (Fig. 1) that was isolated and fully characterized by IR and NMR spectroscopy in our previous work [20]. Im1 represented a key intermediate for the synthesis of pharmacologically relevant compounds endowed with antiproliferative, chelating and GPER-agonistic properties [27,28,29].
In order to extend our knowledge on the reactivity of weak Vilsmeier reagent towards cyclic thioureas, we studied the condensation of 1 with acyl chlorides a-l (Fig. 2) in the presence of N,N-dimethylacetamide (DMA), a DMF homologue. As reported in Fig. 2, the acyl chloride reagents included (cyclo)aliphatic (a,b), variously substituted benzoyl (c–j) and heteroaromatic substructures (k,l) to properly evaluate the effect of different electronic and steric properties on the reaction outcome.
Results and discussion
Reactivity of thiourea 1 with acyl chlorides
The replacement of DMF with its homologous DMA deeply affected the reaction outcome. Despite the in situ condensation of DMA and benzoyl chloride can afford the corresponding acyloxyiminium salt [21], the reaction of 1 with benzoyl chlorides a–l in DMA did not allow the isolation of iminium salt Im2, but led to the formation of mono- or di-acylthioureas (compounds 2 and 3; Scheme 1), depending on the nature of the acylating agent. Thus, (cyclo)alkyl carbonyl chlorides, as well as benzoyl chlorides bearing electron withdrawing groups (i.e. halo or nitro groups), led to the formation of the mono-acylated derivatives 2a–h,j in moderate-to-good yields (Scheme 1). Conversely, under the same reaction conditions, the condensation of 1 with one equivalent of 4-anisoyl chloride i, 2-furoyl chloride k and 2-thenoyl chloride l allowed the isolation of symmetric di-acylated thioureas 3i,k,l in 12%, 16% and 37% yields, respectively (Scheme 1). According to the literature [30], the synthesis of mono-acylated thiourea derivatives is considered problematic, being the formation of the di-acylated compounds favoured also when acyl chlorides were used as limiting reagents. In fact, the only procedure reported in the literature for the synthesis of compound 2c is based on a two-step intramolecular cyclization of 2-hydroxyethyl-thiocarbamides [31].
Mechanistic considerations and hypothesis
In the tested synthetic conditions (i.e. acyl chlorides, DMA) thiourea 1 could react with two electrophilic species (namely, acyl chlorides and weak Vilsmeier reagents II) to form the mono-acylated compounds 2 (Scheme 2). In fact, as previously reported [21], the in situ condensation of DMA and benzoyl chloride led the formation of the corresponding acyloxyiminium salt that has been characterized by proton NMR and IR spectroscopy. The acyl chloride reagent could directly condense with 1 (path a, Scheme 2) or, as observed with DMF [20], react with DMA to afford the weak Vilsmeier reagents II. Out of the two electrophilic centres of intermediates II (i.e. ester carbonyl and iminium carbon), the nucleophilic nitrogen atom of 1 would selectively attack the ester carbonyl group (path b, Scheme 2), as the methyl group would hinder the iminium carbon preventing the formation of Im2 (path c, Scheme 2).
To define whether thiourea acylation would occur via path (a) or (b) (Scheme 2), the calculated partial charges distribution (semi-empirical calculations, AM1 method) of acyl chlorides d, f and i was compared with that of the corresponding intermediates II (Fig. S30, Supporting information). The prediction indicated that the ester carbonyl of intermediates II would be more electrophilic than the acyl chloride carbonyl, thus suggesting II as the prevalent acylating species in the tested conditions.
As experimentally observed, the nature of acyl chloride reagents affects the acylation of thiourea 1 leading to the formation of compounds 2 or 3 (Scheme 3). The different reaction outcome would depend on the reactivity of 2 towards intermediates II (assumed to be the prevalent acylating species) in terms of (i) nucleophilicity of the mono-acylated compound or (ii) energetic content of intermediate III (Scheme 3).
Despite the condensation of 1 with acyl chlorides h and l led to different outcomes (namely, formation of mono- or di-acylthiourea compounds), the calculated partial charge of the thiourea nitrogen appears to be similar in the mono-acylated derivatives 2h and 2l (0.322 for 2h; 0.323 for 2l). Conversely, the calculated energy value of IIIh (Fig. 3, E = 169.21 kcal/mol) was found to be significantly higher than that predicted for IIIl (Fig. 3, E = 100.21 kcal/mol), thus suggesting that the stability of tetrahedral intermediate III would affect the outcome of the acylation reaction more than the nucleophilicity of the mono-acylated derivative.
Synthesis of asymmetric di-acylated thioureas
The chemical accessibility to mono-acylated thioureas prompted us to evaluate their value as useful synthons for the preparation of unreported asymmetric di-acylthioureas. Thus, mono-benzoyl compound 2c was selected as representative derivative of the mono-acylated series and condensed with acyl chlorides a,b,d–f,k,l endowed with different electronic and steric properties (Scheme 4). As detailed in Table 1, asymmetric di-acylthioureas 4–10 were obtained through two different synthetic protocols to take into consideration the different reactivity of acyl chlorides towards the mono-acylated thiourea. In particular, the acylation of 2c with (hetero)aroyl chlorides d–f,k,l was carried out using pyridine as solvent as previously reported [27]. These experimental conditions proved to be ineffective for the preparation of derivatives 4 and 5 which were obtained by reacting a and b with 2c in DMF in the presence of triethylamine (TEA). Compounds 4–10 were isolated in moderate-to-good yields, as detailed in Table 1.
Antiproliferative evaluation
The cytotoxic properties of acylthioureas 2c and 4–10 were preliminary assessed against MCF-7 (breast cancer) and SKOV-3 (ovarian cancer) cell lines by MTT assay (Table 2). At the tested concentration (10 μM), all compounds were devoid of any antiproliferative activity against the selected cell lines, being the mean growth percentage values higher than 82.46%. These data supported the lack of cytotoxicity for the tested compounds and their potential value in different therapeutic areas, other than antitumor agents.
Pharmacokinetic properties and drug-likeness prediction
To evaluate the pharmaceutical relevance of the prepared derivatives, the pharmacokinetics properties and the drug-likeness of compounds 2–10 were calculated by SwissADME [32]. As detailed in Table 3, the calculated physicochemical parameters (i.e. LogP range: − 0.7 to + 5; MW range: 150–500 g/mol; TPSA range: 20–130 Å2; Fraction Csp3 range: 0.5–1; number of rotatable bonds: 0–9) indicated a good oral bioavailability for acylthioureas 2. Furthermore, the predicted gastrointestinal (GI) absorption for the mono-acylated thioureas was high without any blood–brain barrier (BBB) penetration. According to the calculations, all derivatives 2 should not be able to inhibit cytochrome (CYP) isoforms 2C9, 2D6 and 3A4, whereas CYP1A2 would be blocked by (hetero)aroyl derivatives 2c–h, j but not by the (cyclo)alkylcarbonyl compounds 2a,b. Furthermore, the presence of a chlorine atom at position 4 of the benzoyl substructure would be related to the inhibition of CYP2C19 enzyme. The drug-like properties of derivatives 2 appear to be good, as no violations of the Lipinski rules were detected. Acylthioureas 2 did not showed any pan assay interference compound (PAINS) alerts, whereas the presence of the thiocarbonyl functionality (and of the nitro group for 2h) was spotted as problematic fragment(s) according to the Brenk filters [33]. Noteworthily, this lead-likeness violation focussed on the physicochemical boundaries defining a good lead (i.e. a small and hydrophilic compound suitable for optimization) and does not undermine the pharmaceutical potential of the compound series. A molecular weight (MW) lower than 250 g/mol has been estimated to be a limitation of the lead-likeness (i.e. a suitability for optimization) of derivatives 2a–g, as implemented by Teague and co-workers [34].
According to the calculation carried out on di-acylthioureas 3–10 (Table 4), the physicochemical parameters (i.e. LogP; MW; TPSA; fraction Csp3 and number of rotatable bonds) of compounds 4 and 5 were considered suitable for oral bioavailability while the elevated degree of instauration (fraction Csp3 lower than 0.25) would negatively affect the oral absorption of derivatives 3,6–10. Compounds 3–10 would act as inhibitors of different CYP isoforms (Table 4), being the 2C9 and 2C19 enzymes the most affected. Additionally, all compounds would be endowed with high GI absorption, whereas the penetration of the BBB would be related to the presence of a chloro-substituted benzoyl substructure. Likewise the mono-acylated precursor 2c, compounds 3–10 displayed good drug-like properties as indicated by the absence of Lipinski violations. The di-acylated derivatives were predicted to be valuable lead compounds for further chemical optimization, despite the high logP values (greater than 3.5) of derivatives 6–8 and the MW value of 3i exceeding the 350 g/mol cut-off. The presence of the thiocarbonyl functionality would represent a lead-likeness limitation, as highlighted by the Brenk alert value.
Conclusions
The condensation of cyclic thiourea 1 with acyl chlorides in DMA was identified as a novel, single-step, effective procedure for the preparation of mono-acylated derivatives 2. The developed procedure emerged to be versatile and allowed the preparation of mono (cyclo)alkyl carbonyl, benzoyl and heteroaryl thiourea derivatives. The identified conditions led to the synthesis of mono-acylated imidazolidine-2-thione compounds whose preparation proved to be difficult in other conditions reported in the literature; in fact, the mono-acyl derivatives emerged to be more reactive than the parent thioureas towards the acylating agent. The chemical accessibility to mono-benzoyl thiourea 2c allowed the preparation of a small library of unreported asymmetric di-acylthioureas. The obtained derivatives 4–10 were devoid of any cytotoxicity in preliminary MTT screening carried out against MCF-7 and SKOV-3 cancer cells. The lack of cytotoxicity represents the starting point for future studies focussed on the evaluation of the pharmaceutical potentials of these compounds in therapeutic areas other than anticancer agents. Furthermore, in silico studies predicted for mono- and di-acylated thioureas good drug-like and pharmacokinetics properties that further support the potential of compounds 2–10 in the medicinal chemistry area.
Experimental section
Chemistry
Commercially available thiourea 1 and acyl chlorides a-l were purchased by Alfa-Aesar and Sigma-Aldrich. DMF, DMA and pyridine were reagent grade and were dried on molecular sieves (5 Å 1/16" inch pellets). Unless otherwise stated, all commercial reagents were used without further purification. Organic solutions were dried over anhydrous sodium sulphate. Thin-layer chromatography (TLC) system for routine monitoring the course of parallel reactions and confirming the purity of analytical samples employed aluminium-backed silica gel plates (Merck DC-Alufolien Kieselgel 60 F254). DCM or DCM/methanol (9:1) were used as a developing solvent and detection of spots was made by UV light and/or by iodine vapours. Melting points were determined on a Fisher-Johns apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Varian Gemini, Bruker DPX-300 or JEOL JNM-ECZR instrument; chemical shifts were reported in d (ppm) units relative to the internal reference tetramethylsilane and the splitting patterns were described as follows: s (singlet), bs (broad singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). The first-order values reported for coupling constants J were given in Hz. Elemental analyses were performed by an EA1110 Analyzer, Fison Instruments (Milan).
Synthesis of compounds 2 and 3
A dry DMA (8 ml) solution of 1 (1.04 g, 10 mmol) and the proper acyl chloride (10 mmol) were stirred at 90 °C for 30 min. After cooling to rt, water (30 ml) and 1 M K2CO3 solution (pH 8) were added. The mixture was extracted with DCM (2 × 15 ml) and the pooled organic phases were washed with water (1 × 10 ml), dried with anhydrous Na2SO4 and concentrated under reduced pressure. The crude material was purified by crystallization from the proper solvent or solvent mixture.
3-Methyl-1-(2-thioxoimidazolidin-1-yl)butan-1-one (2a)
White solid; yield 44%; mp: 135–137 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 0.90 (d, J = 6.6 Hz, 6H, 2 × CH3); 2.04–2.19 (m, 1H, CH); 3.20 (d, J = 6.9 Hz, 2H, CH2–CO); 3.40–3.49 (m, 2H, CH2N); 3.95–4.04 (m, 2H, CH2N); 9.66 (bs, 1H, NH deuterable). 13C NMR (101 MHz, DMSO-D6): δ 22.3, 25.2, 44.8, 46.9, 173.1, 179.2. Anal. Calcd for C8H14N2OS: C, 51.58; H, 7.58; N, 15.04; S, 17.21. Found: C, 51.28; H, 7.52; N, 15.14; S; 17.23.
Cyclopropyl(2-thioxoimidazolidin-1-yl)methanone (2b)
White solid; yield: 47%; mp: 143–145 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 0.88–0.97 (m, 4H, 2 × CH2 cycloprop.); 3.41–3.51 (m, 2H, CH2N); 3.94–4.03 (m, 2H, CH2N); 4.10–4.21 (m, 1H, CHCO); 9.73 (bs, 1H, NH exchangeable). 13C NMR (101 MHz, DMSO-D6): δ 10.2, 13.5, 40.3, 47.3, 174.7, 179.5. Anal. Calcd for C7H10N2OS: C, 49.39; H, 5.98; N, 16.45; S, 18.83. Found: C, 49.33; H, 5.69; N, 16.53; S, 18.66.
Phenyl(2-thioxoimidazolidin-1-yl)methanone (2c)
Yellow solid; yield 32%; mp: 152–154 °C (neat DCM). 1H NMR (400 MHz, DMSO-D6): δ 3.54–3.63 (m, 2H, CH2N); 4.06–4.15 (m, 2H, CH2N); 7.32–7.41 (m, 2H, arom. H); 7.43–7.55 (m, 2H, arom. H); 9.74 (bs, 1H, NH deuterable). 13C NMR (101 MHz, DMSO-D6): δ 41.2, 48.1, 127.4, 128.8, 131.0, 135.7, 171.2, 180.1. Anal. Calcd for C10H10N2OS: C, 58.23; H, 4.89; N, 13.58; S, 15.55. Found: C, 58.41; H, 4.70; N, 13.77; S, 15.35.
(2-chlorophenyl)(2-thioxoimidazolidin-1-yl)methanone (2d)
White solid; yield 23%; mp: 195–197 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): 3.54–3.63 (m, 2H, CH2N); 4.12–4.21 (m, 2H, CH2N); 7.27–7.44 (m, 4H, arom. H); 9.82 (bs, 1H, NH deuterable). 13C NMR (101 MHz, DMSO-D6): δ 40.9, 46.5, 126.6, 128.7, 129. 8, 130.5, 136.5, 167.1, 178.7. Anal. Calcd for C10H9ClN2OS: C, 49.90; H, 3.77; N, 11.64; S, 13.32. Found: C, 49.85; H, 3.68; N, 11.68; S, 13.42.
(3-chlorophenyl)(2-thioxoimidazolidin-1-yl)methanone (2e)
White solid: yield 78%; mp: 134–137 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 3.54–3.63 (m, 2H, CH2N); 4.06–4.15 (m, 2H, CH2N); 7.35–7.61 (m, 4H, arom. H); 9.84 (bs, 1H, NH deuterable). Anal. Calcd for C10H9ClN2OS: C, 49.90; H, 3.77; N, 11.64; S, 13.32. Found: C, 50.27; H, 3.82; N, 11.86; S, 13.53.
(4-chlorophenyl)(2-thioxoimidazolidin-1-yl)methanone (2f)
Yellow solid; yield 55%; mp: 155–157 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 3.54–3.62 (m, 2H, CH2N); 4.06–4.16 (m, 2H, CH2N); 7.41–7.47 (m, 2H, arom. H); 7.50–7.56 (m, 2H, arom. H); 9.80 (bs, 1H, NH deuterable). Anal. Calcd for C10H9ClN2OS: C, 49.90; H, 3.77; N, 11.64; S, 13.32. Found: C, 50.08; H, 3.40; N, 11.53; S, 13.06.
(4-fluorophenyl)(2-thioxoimidazolidin-1-yl)methanone (2g)
White solid; yield 26%; mp: 165–167 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 3.53–3.62 (m, 2H, CH2N); 4.06–4.14 (m, 2H, CH2N); 7.15–7.25 (m, 2H, arom. H); 7.54–7.64 (m, 2H, arom. H); 9.77 (bs, 1H, NH, deuterable). Anal. Calcd for C10H9N2OSF: C, 53.56; H, 4.05; N, 12.49; S, 14.30. Found: C, 53.53; H, 4.05; N, 12.67; S, 14.59.
(4-Nitrophenyl)(2-thioxoimidazolidin-1-yl)methanone (2h)
Yellow solid; yield 70%; mp: 179–181 °C (DCM/MeOH). 1H NMR (200 MHz, CDCl3): δ 3.57–3.70 (m, 2H, CH2N); 4.08–4.278 (m, 2H, CH2N); 7.67–7.78 and 8.17–8.29 (m, 4H, arom. H); 9.97 (bs, 1H, NH, deuterable). Anal. Calcd for C10H9N3O3S: C, 47.80; H, 3.61; N, 16.72; S, 12.76. Found: C, 47.50; H, 3.50; N, 16.62; S, 12.52.
(3,4-Dichlorophenyl)(2-thioxoimidazolidin-1-yl)methanone (2j)
White solid; yield 70%; mp: 150–155 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 3.45–3.64 (m, 2H, CH2N); 4.06–4.15 (m, 2H, CH2N); 7.43–7.50 (m, 1H, arom. H); 7.62–7.68 (m, 1H, arom. H); 7.75–7.80 (m, 1H, arom. H); 9.91 (bs, 1H, NH, deuterable). 13C NMR (101 MHz, DMSO-D6): δ 41.3, 47.8, 128.6, 129.9, 130.1, 130.6, 133.2, 136.3, 168.6, 179.6. Anal. Calcd for C10H8N2Cl2OS: C, 43.65; H, 2.93; N, 10.18; S, 11.65. Found: C, 43.44; H, 2.91; N, 10.44; S, 11.82.
(2-Thioxoimidazolidine-1,3-diyl)bis((4-methoxyphenyl)methanone) (3i)
Yellow solid; yield 12%; mp: 198–201 °C (DCM/MeOH). 1H NMR (400 MHz, CDCl3): δ 3.83 (s, 6H, 2 × OCH3); 4.16–4.20 (m, 4H, 2 × CH2N); 6.81–6.90 (m, 4H, arom. H); 7.65–7.72 (m, 4H, arom. H). Anal. Calcd for C19H18N2O4S: C, 61.61; H, 4.90; N, 7.56; S, 8.65. Found: C, 61.81; H, 4.79; N, 7.62; S, 8.75.
(2-Thioxoimidazolidine-1,3-diyl)bis(furan-2-ylmethanone) (3k)
Yellow solid; yield 16%; mp: 141–144 °C (DCM/MeOH). 1H NMR (200 MHz, CDCl3): δ 4.07- 4.23 (m, 4H, 2 × CH2N); 6.42–6.58 (m, 2H, H(4)-furan); 7.12–7.25 (m, 2H, H(3)-furan); 7.43–7.60 (m, 2H, H(5)-furan). Anal. Calcd for C13H10N2O4S: C, 53.79; H, 3.47; N, 9.65; S, 11.04. Found: C, 53.70; H, 3.47; N, 9.81; S, 11.36.
(2-Thioxoimidazolidine-1,3-diyl)bis(thiophen-2-ylmethanone) (3l)
Yellow solid; yield 37%; mp: 195–197 °C (DCM/MeOH). 1H NMR (400 MHz, DMSO-D6): δ 4.18–4.21 (m, 4H, 2 × CH2N); 7.17–7.24 (m, 2H, H4-thiophene); 7.86–7.94 (m, 2H, 2 × H5-thiophene); 7.98–8.05 (m, 2H, 2 × H3-thiophene). 13C NMR (101 MHz, DMSO-D6): δ 47.1, 128.1, 134.9, 135.4, 136.8, 164.6, 179.5. Anal. Calcd for C13H10N2O2S3: C, 48.43; H, 3.13; N, 8.69; S, 29.83. Found: C, 48.97; H, 3.10; N, 8.74; S, 29.62.
Synthesis of compounds 4 and 5
To a dry DMF (10 ml) solution of 2c (0.35 g, 1.7 mmol), TEA (266 ml, 1.9 mmol) and the proper acyl chloride (1.9 mmol) were added sequentially. The reaction mixture was stirred at rt for 45 min and then heated at 40 °C for 15 min. After dilution with water (40 ml) the mixture was kept at 4 °C for 2 hours and the precipitated solid was filtered. The crude material was purified by crystallization from DCM/MeOH mixture.
1-(3-Benzoyl-2-thioxoimidazolidin-1-yl)-3-methylbutan-1-one (4)
Yellow solid; yield 24%; mp: 100–103 °C. 1H NMR (400 MHz, CDCl3): δ 0.92–1.01 (m, 6H, 2 × CH3); 2.16–2.30 (m, 1H, CH); 3.09–3.16 (m, 2H, CH2–CO); 4.00–4.10 (m, 2H, CH2N); 4.11–4.22 (m, 2H, CH2N); 7.36–7.46 (m, 2H, arom. H); 7.48–7.55 (m, 1H, arom. H); 7.62–7.69 (m, 2H, arom. H). 13C NMR (101 MHz, CDCl3): δ 22.6, 25.2, 44.6, 44.8, 47.1, 128.3, 129.2, 132.4, 134.8, 172.4, 174.6, 178.6. Anal. Calcd for C15H18N2O2S: C, 62.04; H, 6.25; N, 9.65; S, 11.04. Found: C, 61.86; H, 6.05; N, 9.84; S, 11.07.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(cyclopropyl)methanone (5)
White solid; yield 18%; mp: 136–137 °C. 1H NMR (300 MHz, CDCl3): δ 0.99–1.04 (m, 2H, CH2cyc); 1.18–1.21 (m, 2H, CH2cyc); 3.67–3.70 (m, 1H, CHCO); 4.07–4.14 (m, 4H, 2 × CH2N); 7.39–7.68 (m, 5H, arom. H). Anal. Calcd for C14H14N2O2S: C, 61.29; H, 5.14; N, 10.21; S, 11.68. Found: C, 61.32; H, 4.91; N, 10.20; S, 12.11.
Synthesis of compounds 6–10
An anhydrous pyridine (10 ml) solution of 2c (0.325 g, 1.57 mmol) and the proper acyl chloride (1.9 mmol) were heated at 90 °C for 0.5 h (for 6, 2 h). After dilution with water (40 ml) the mixture was kept at 4 °C for 2 h and the precipitated solid was filtered. The crude material was purified by crystallization from DCM/MeOH mixture.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(2-chlorophenyl)methanone (6)
Yellow solid; yield 16%; mp: 131–133 °C. 1H NMR (400 MHz, DMSO-D6): δ 4.31–4.38 (m, 4H, 2 × CH2N); 7.44–7.53 (m, 7H, arom. H); 7.59–7.62 (m, 2H, arom. H). Anal. Calcd for C17H13N2O2SCl: C, 59.21; H, 3.80; N, 8.12; S, 9.30. Found: C, 59.34; H, 3.78; N, 7.99; S, 10.01.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(3-chlorophenyl)methanone (7)
Ivory solid; yield 74%; mp: 150–151 °C. 1H NMR (200 MHz, CDCl3): δ 4.13–4.30 (m, 4H, 2 × CH2N); 7.25–7.68 (m, 9H, arom. H). Anal. Calcd for C17H13ClN2O2S: C, 59.21; H, 3.80; N, 8.12; S, 9.30. Found: C, 59.25; H, 4.02; N, 8.35; S, 8.52.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(4-chlorophenyl)methanone (8)
Yellow solid; yield 35%; mp: 201–203 °C. 1H NMR (400 MHz, CDCl3): δ 4.21–4.25 (m, 4H, 2 × CH2N); 7.29–7.64 (m, 9H, arom. H). 13C NMR (101 MHz, CDCl3): δ 45.6, 128.2, 128.5, 129.1, 130.6, 132.4, 132.9, 134.4, 138.5, 171.1, 172.0, 178.9. Anal. Calcd for C17H13ClN2O2S: C, 59.21, H, 3.80, N, 8.21, S, 9.30. Found: C, 60.86, H, 3.75, N, 8.65, S, 8.16.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(furan-2-yl)methanone (9)
Yellow solid; yield 36%; mp: 175–176 °C. 1H NMR (400 MHz, CDCl3): δ 4.24–4.29 (m, 4H, 2 × CH2N); 6.55–6.58 (m, 1H, H furan); 7.25–7.72 (m, 7H, Arom. H + H furan). 13C NMR (101 MHz, CDCl3): δ 45.5, 46.0, 112.7, 119.9, 128.2, 129.1, 132.3, 134.6, 145.9, 147.1, 160.5, 171.9, 178.4. Anal. Calcd for C15H12N2O3S: C, 59.99; H, 4.03; N, 9.33; S, 10.68. Found: C, 59.86; H, 4.25; N, 9.59; S, 9.18.
(3-Benzoyl-2-thioxoimidazolidin-1-yl)(thiophen-2-yl)methanone (10)
Yellow solid; yield 38%; mp: 186–188 °C. 1H NMR (400 MHz, CDCl3): δ 4.14–4.31 (m, 4H, 2 × CH2N); 6.98–7.09 (m, 1H, H thiophen); 7.31–7.75 (m, 7H, arom. H and H thiophen). 13C NMR (101 MHz, CDCl3): δ 45.8, 46.1, 127.6, 128.1, 129.1, 132.3, 133.8, 134.6, 134.9, 136.8, 165.1, 172.0, 179.3. Anal. Calcd for C15H12N2O2S2: C, 56.94; H, 3.82; N, 8.85; S, 20.27. Found: C, 56.95; H, 4.12; N, 9.00; S, 18.19.
Biology
To perform MTT assay, SKOV-3 (ovarian adenocarcinoma, ATCC, Manassas, VA) and MCF-7 (breast adenocarcinoma, Biologic Bank and Cell Factory, IRCCS Policlinico San Martino, Genoa, Italy) cell lines were cultured in DMEM (added with 10% FBS, 2 mM Glutamine and 1% penstrep. Reagents were acquired from EuroClone, Milan, Italy) and incubated in humidified conditions at 37 °C with 5% CO2. Chemical compounds were dissolved in DMSO to give a 10 mM stock solution. Then, once diluted in growth medium, they were added to the cultured cells at a final working concentration of 10 μM and incubated for 48 h. At the end of the incubation, 30 μl of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) at a concentration of 2 mg/ml in PBS were added in each well and incubated 4 h. Finally, the surnatant was removed and 100 μl/well of DMSO were added to dissolve the Formazan precipitate. After 20 min, the results were read at 570 nm. Results are expressed as percentage of the control samples where cells have been treated with the same amount of DMSO but without any chemical compound. The assay was repeated three times and a single compound was tested six times. Means and standard deviations were calculated.
Computational calculations
The chemical structures of studied compounds were drawn with MOE2009.10 (builder module) and energy minimization was carried out according to AM1, as implemented in MOE software. The calculations were run on a Linux PC (Intel® processor Core™ i7-2600 CPU@3.40 GHz).
References
Sun C, Huang H, Feng M, Shi X, Zhang X, Zhou P (2006) A novel class of potent influenza virus inhibitors: polysubstituted acylthiourea and its fused heterocycle derivatives. Bioorg Med Chem Lett 16:162–166. https://doi.org/10.1016/j.bmcl.2005.09.033
Saeed A, Batool M (2008) Synthesis and bioactivity of some new 1-tolyl-3-aryl-4-methylimidazole-2-thionesMed. Chem Res 16:143–154. https://doi.org/10.1007/s00044-007-9017-8
Toldy L, Solyom S, Kocka I, Toth G, Toth I (1971) Thiourea derivatives with tuberculostatic action. II. Acylthiocarbamides. Acta Chim Acad Sci Hung 69:221–227
Plutín AM, Alvarez A, Mocelo R, Ramos R, Castellano EE, da Silva MM, Villarreal W, Pavan FR, Meira C, Rodrigues FJS, Moreira DR, Soares MB, Batista AA (2017) Antitumor activity of Pd(II) complexes with N, S or O, S coordination modes of acylthiourea ligands. Polyhedron 132:70. https://doi.org/10.1016/j.poly.2020.114543
Misra VS, Pandey RN, Dua PR (1979) Synthesis of N-aryl-N’-[2-phenyl-3-quinazolino(3H)-4-one] acylthiourea derivatives as anticonvulsants. Pol J Pharmacol Pharm 31:161–167
Ranise A, Bondavalli F, Bruno O, Schenone S, Donnoli D, Parrillo C, Cenicola ML, Rossi F (1991) 1-Acyl-, 3-acyl- and 1,3-diacyl-3-furfuryl-1-phenylthioureas with platelet antiaggregating and other activities. Farmaco 46:1203–1216
Ranise A, Bondavalli F, Bruno O, Schenone S, Losasso C, Costantino M, Cenicola ML, Donnoli D, Marmo E (1991) 3,3-Disubstituted 1-acyl-1-phenylthioureas with platelet antiaggregating and other activities. Farmaco 46:317–338
Ranise A, Spallarossa A, Bruno O, Schenone S, Fossa P, Menozzi G, Bondavalli F, Mosti L, Capuano A, Mazzeo F, Falcone G, Filippelli W (2003) Synthesis of N-substituted-N-acylthioureas of 4-substituted piperazines endowed with local anaesthetic, antihyperlipidemic, antiproliferative activities and antiarrythmic, analgesic, antiaggregating actions. Farmaco 58:765–780. https://doi.org/10.1016/S0014-827X(03)00132-0
Roy G, Das D, Mugesh G (2007) Bioinorganic chemistry aspects of the inhibition of thyroid hormone biosynthesis by anti-hyperthyroid drugs. Inorg Chim Acta 360:303–316. https://doi.org/10.1016/j.ica.2006.07.052
Rodger A, Patel KK, Sanders KJ, Datt M, Sacht C, Hannon M (2002) Anti-tumour platinum acylthiourea complexes and their interactions with DNA. J Chem Soc Dalton Trans 19:3656–3663
Hallur G, Jimeno A, Dalrymple S, Zhu T, Jung MK, Hidalgo M, Isaacs JT, Sukumar S, Hamel E, Khan SR (2006) Benzoylphenylurea sulfur analogues with potent antitumor activity. J Med Chem 49:2357–2360. https://doi.org/10.1021/jm051261s
Barolli JP, Maia PIS, Colina-Vegas L, Moreira J, Plutin AM, Mocelo R, Deflon VM, Cominetti MR, Camargo-Mathias MI, Batista AA (2017) Heteroleptic tris-chelate ruthenium(II) complexes of N, N-disubstituted-N′-acylthioureas: synthesis, structural studies, cytotoxic activity and confocal microscopy studies. Polyhedron 126:33–41. https://doi.org/10.1016/j.poly.2017.01.002
Haerter M, Kosemund D, Delbeck M, Kalthof B, Wasnaire P, Suessmeier F, Lustig K (2016) Heterocyclylmethyl-thienouracile as antagonists of adenosine-A2B receptor and their preparation. WO2016150901 A1 2016-09-29
Beeley NRA, Behan DP, Chalmers DT, Menzaghi F, Strah-Pleynet S (2001) Preparation of imidazolidinethiones and imidazodithiazoles as inverse agonists of G protein-coupled receptor 6 (GPR6). WO2001062765 A2 2001–08–30
Jomaa MY, Isab AA (2019) Anticancer activity of cis-diamine platinum(II) complexes of thiones. United States, US20190125717 A1 2019-05-02
Wazeer MIM, Isab AA, Fettouhi M (2007) New cadmium chloride complexes with imidazolidine-2-thione and its derivatives: X-ray structures, solid state and solution NMR and antimicrobial activity studies. Polyhedron 26:1725–1730. https://doi.org/10.1016/j.poly.2006.12.022
Aulakh JK, Lobana TS, Sood H, Arora DS, Garcia-Santos I, Kaur M, Jasinski JP (2020) Synthesis, structures, and novel antimicrobial activity of silver(I) halide complexes of imidazolidine-2-thiones. Polyhedron. https://doi.org/10.1016/j.poly.2019.114235
Araki K, Murata T, Gunjima K, Nakakura N, Shimojo E, Arnold C, Hempel W, Jans D, Malsam O, Waibel JM (2003) Preparation of pyridinecarboxamides as pesticides. WO2003097604 A1 2003-11-27
Takaoka D (2009) Preparation of heterocyclic compounds as arthropod control agents. JP2009073776 A 2009-04-09
Ranise A, Cesarini S, Spallarossa A, Sancassan F, Bondavalli F, Bruno O, Schenone S, Menozzi G, Fossa P, Mosti L (2007) Unprecedented one-pot stereoselective synthesis of Knoevenagel-type derivatives via in situ condensation of N-methyleniminium salts of ethylenethiourea and ethyleneurea with active methylene reagents. Synthesis 16:2495–2502. https://doi.org/10.1055/s-2007-983811
Bottomley WE, Boy GV (1980) Acylation of tertiary amides. Formation of 1-acyloxyiminium salts and 1-acyloxyenamines. J Chem Soc Chem Commun 16:790–791. https://doi.org/10.1039/C39800000790
Hall HK (1956) Kinetics of reactions of acyl chlorides. IV.1 Solvolysis of acyl halides in dimethylformamide. J Am Chem Soc 78:2717–2719
Bredereck H, Gompper R, Klemm K, Rempfer H (1959) Formamid-Reaktionen, XIV. Reaktionen von Säureamid-Acylhalogenid-Addukten: Darstellung substituierter Amidine und Amidrazone. Chem Ber 92:837–849
Horning DE, Muchowski JM (1967) The dimethyl formamide–acyl halide complex and its application to the synthesis of acyl azides. Can J Chem 45:1247–1251
Barluenga J, Campos PJ, Gonzalez-Nuñez E, Asensio G (1985) General method for the formylation of alcohols with dimethylformamide: an extension of the Vilsmeier-Haack reaction. Synthesis 4:426–428. https://doi.org/10.1055/s-1985-31228
Zarei M (2012) Utilization of DMF-PhCOCl adduct as an acid activator in a new and convenient method for preparation of b-lactams. Bull Chem Soc Jpn 85:360–368
Cesarini S, Spallarossa A, Ranise A, Schenone S, Rosano C, La Colla P, Sanna G, Busonera B, Loddo R (2009) N-Acylated and N, N′-diacylated imidazolidine-2-thione derivatives and N, N′-diacylated tetrahydropyrimidine-2(1H)-thione analogues: synthesis and antiproliferative activity. Eur J Med Chem 44:1106–1118. https://doi.org/10.1016/j.ejmech.2008.06.010
Lappano R, Rosano C, Santoll MF, Pupo M, De Francesco EM, De Marco P, Ponassi M, Spallarossa A, Ranise A, Maggiolini M (2012) Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr Cancer Drug Targets 12:531–542. https://doi.org/10.2174/156800912800673284
Caneva C, Alfei S, De Maria M, Ibba C, Delogu I, Spallarossa A, Loddo R (2015) Synthesis and biological evaluation of (acyl)hydrazones and thiosemicarbazones obtained via in situ condensation of iminium salts with nitrogen-containing nucleophiles. Mol Divers 19:669–684. https://doi.org/10.1007/s11030-015-9597-z
Peet NP, Malecha J, Le Tourneau ME, Sunder S (1989) Preparation of imidazo[2,1-b]quinazolin-5(3H)-ones and related tricyclic systems using a novel, double displacement reaction. J Heterocyclic Chem 26:257–264. https://doi.org/10.1002/JHET.5570260147
Zade MN, Katiya MM, Deotale VD, Sontakke MM, Dhonde MG, Berad BN (2018) Synthesis of thiazol, thiazinan, thiadiazin, thiazolidin, triazine, thioxo-pyrimidin and thioxo-imidazolidine by inter-intra molecular cyclization. Indian J Chem 57B:1493–1500
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Brenk R, Schipani A, James D, Krasowski A, Gilbert IH, Frearson J, Wyatt PG (2008) Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 3:435–444. https://doi.org/10.1002/cmdc.200700139
Teague S, Davis A, Leeson P, Oprea T (1999) The design of leadlike combinatorial libraries. Angew Chem Int Ed Engl 38:3743–3748
Delaney JS (2004) ESOL: estimating aqueous solubility directly from molecular structure. J Chem Inf Model 44:1000–1005
Acknowledgements
A.Sp. acknowledge Dr. Martina Giuffra for helpful discussion and support. This research was funded by University of Genova (FRA grant). The work of M.P. and C.R. has been partially supported by a grant from the Italian Ministry of Health (Ricerca Corrente 2021).
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scarsi, A., Ponassi, M., Brullo, C. et al. Mono- and di-acylated imidazolidine-2-thione derivatives: synthesis, cytotoxicity evaluation and computational studies. Mol Divers 27, 1285–1295 (2023). https://doi.org/10.1007/s11030-022-10487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10487-5