Abstract
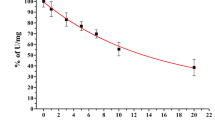
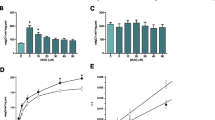
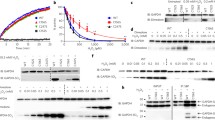
The mitochondrial electron transport chain (ETC) contains thiol groups (−SH) which are reversibly oxidized to modulate ETC function during H2O2 overproduction. Since deleterious effects of H2O2 are not limited to –SH oxidation, due to the formation of other H2O2-derived species, some processes like lipoperoxidation could enhance the effects of H2O2 over ETC enzymes, disrupt their modulation by –SH oxidation and increase superoxide production. To verify this hypothesis, we tested the effects of H2O2 on ETC activities, superoxide production and iron mobilization in mitochondria from lipoperoxidation-resistant native yeast and lipoperoxidation-sensitized yeast. Only complex III activity from lipoperoxidation-sensitive mitochondria exhibited a higher susceptibility to H2O2 and increased superoxide production. The recovery of ETC activity by the thiol reductanct β-mercaptoethanol (BME) was also altered at complex III, and a role was attributed to lipoperoxidation, the latter being also responsible for iron release. A hypothetical model linking lipoperoxidation, increased complex III damage, superoxide production and iron release is given.
Similar content being viewed by others
References
Avéret N, Fitton V, Bunoust O, Rigoulet M, Guérin B (1998) Mol Cell Biochem 184:67–79
Beal MF (2003) Ann NY Acad Sci 991:120–131
Boveris A, Cadenas E (1975) FEBS Lett 54:311–314
Breuer W, Epsztejn S, Cabantchik ZI (1995) J Biol Chem 270:24209–24215
Buege JA, Aust D (1978) Methods Enzymol 52:302–310
Cadenas E, Davies KJA (2000) Free Radic. Biol Méd 29:222–230
Cardoso SM, Pereira C, Oliveira R (1999) Free Radic. Biol Méd 26:3–13
Chen Y-R, Gunther MR, Mason RP (1999) J Biol Chem 274:3308–3314
Chen H, Zheng C, Zhang Y, Chang Y-Z, Qian ZM, Shen X (2006) Int J Biochem Cell Biol 38:1402–1416
Cortés-Rojo C, Calderón-Cortés E, Clemente-Guerrero M, Manzo-Avalos S, Uribe S, Boldogh I, Saavedra-Molina A (2007) Free Radic Res 41:1212–1223
Cortés-Rojo C, Calderón-Cortés E, Clemente-Guerrero M, Estrada-Villagómez M, Manzo-Avalos S, Mejía-Zepeda R, Boldogh I, Saavedra-Molina A (2009) J Bioenerg Biomembr 41:15–28
Dikalov S, Losik T, Arbiser JL (2008) Biochem Pharmacol 76:589–596
Dimroth P, Kaim G, Matthey U (2000) J Exp Biol 203:51–59
Forman HJ, Fukuto JM, Torres M (2004) Am J Physiol Cell Physiol 287:C246–C256
Gornall AG, Bardawill CJ, David MM (1949) J Biol Chem 177:751–765
Guérin B, Labbe P, Somlo M (1979) Methods Enzymol 55:149–159
Hallberg EM, Shu Y, Hallberg RL (1993) Mol Cell Biol 13:3050–3057
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, New York
Holman RT (1954) In: Holman RT, Lundberg WO, Malkin T (eds) Progress in the chemistry of fats and other lipids: autooxidation of fats and related substances. Academic Press, New York, pp 51–98
Hondorp ER, Matthews RG (2004) PLoS Biol 2:e336
Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP (2007) J Biol Chem 282:22040–22051
Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP (2008) J Biol Chem 283:24801–24815
Jacob C, Holme AL, Fry FH (2004) Org Biomol Chem 2:1953–1956
Jakob U, Eser M, Bardwell JC (2000) J Biol Chem 275:38302–38310
James AM, Cochemé HM, Smith RAJ, Murphy MP (2005) J Biol Chem 280:21295–21312
Jang S, Imlay JA (2007) J Biol Chem 282:929–937
Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK (2000) J Biol Chem 275:26096–26101
Jones DP (2008) Am J Physiol Cell Physiol 295:849–868
Kiley PJ, Storz G (2004) PLoS Biol 2:1714–1717
Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG (2000) Anal Biochem 283:214–221
Kim MH, Chung J, Yang JW, Chung SM, Kwag NH, Yoo JS (2003) Korean J Ophthalmol 17:19–28
Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA (2005) J Biol Chem 280:37481–37488
Krause KH (2007) Exp Gerontol 42:256–262
Kwok E, Kosman D (2006) In: Tamàs MJ, Martinoia E (eds) Molecular biology of metal homeostasis and detoxification. From microbes to man: iron in yeast: mechanisms involved in homeostasis. Springer, Berlin, pp 59–100
Lê-Quôc K, Lê-Quôc D, Gaudemer Y (1981) Biochemistry 20:1705–1710
Lin TK, Hughes G, Muratovska A, Blaikie FH, Brookes PS, Darley-Usmar V, Smith RA, Murphy MP (2002) J Biol Chem 277:17048–17056
Longo VD, Liou LL, Valentine JS, Gralla EB (1999) Arch Biochem Biophys 365:131–142
Łukaszewicz-Hussain A, Moniuszko-Jakoniuk J (2004) Polish J Environ Studies 13:397–401
Ly JD, Grubb DR, Lawen A (2003) Apoptosis 8:115–128
Malis CD, Weber PC, Leaf A, Bonventre JV (1999) Proc Natl Acad Sci USA 87:8845–8849
Martin J, Mahlke K, Pfanner N (1991) J Biol Chem 266:18051–18057
Martin CE, Oh C, Jiang Y (2007) Biochim Biophys Acta 1771:271–285
Masini A, Ceccarelli D, Giovannini F, Montosi G, Garuti C, Pietrangelo AJ (2000) J Bioenerg Biomembr 32:175–182
Matsuno-Yagi A, Hatefi Y (1996) J Biol Chem 271:6164–6171
Muller FL, Crofts AR, Kramer DM (2002) Biochemistry 41:7866–7874
Nicholls DG (2005) Cell Calcium 38:311–317
North JA, Spector AA, Buettner GR (1992) J Biol Chem 267:5743–5746
Nulton-Persson AC, Szweda LI (2001) J Biol Chem 276:23357–23361
Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA (2002) Gastroenterology 122:366–375
Schoneich C, Dillinger U, von Bruchhausen F, Asmus KD (1992) Arch Biochem Biophys 292:456–467
Seppet E, Gruno M, Peetsalu A, Gizatullina Z, Nguyen HP, Vielhaber S, Wussling MHP, Trumbeckaite S, Arandarcikaite O, Jerzembeck D, Sonnabend M, Jegorov K, Zierz S, Striggow F, Gellerich FN (2009) Int J Mol Sci 10:2252–2303
Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM, Protect Study Group (2010) Mov Disord 25:1670–1674
Spector A, Wang G-M, Wang R-R (1993) Proc Natl Acad Sci USA 90:7485–7489
Tatsumi T, Kako KJ (1993) Basic Res Cardiol 88:199–211
Turrens JF (2003) J Physiol 552:335–344
Ueda N, Guidet B, Shah SV (1993) Am J Physiol 265:F435–F439
Uribe S, Ramirez J, Peña A (1985) J Bact 161:1195–1200
Vygodina TV, Konstantinov AA (2007) Biochemistry (Mosc) 72:1056–1064
Zheng M, Aslund F, Storz G (1998) Science 279:1718–1721
Zini R, Berdeaux A, Morin D (2007) Free Radic Res 41:1159–1166
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cortés-Rojo, C., Estrada-Villagómez, M., Calderón-Cortés, E. et al. Electron transport chain dysfunction by H2O2 is linked to increased reactive oxygen species production and iron mobilization by lipoperoxidation: studies using Saccharomyces cerevisiae mitochondria. J Bioenerg Biomembr 43, 135–147 (2011). https://doi.org/10.1007/s10863-011-9339-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-011-9339-6