Abstract
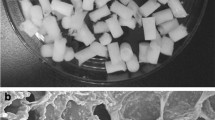
Bioceramic mixtures of tricalcium phosphate (TCP) and hydroxyapatite (HAp) are widely used for bone regeneration because of their excellent cytocompatibility, osteoconduction, and osteoinduction. Therefore, we hypothesized that incorporation of a mixture of TCP and HAp in microsphere-based scaffolds would enhance osteogenesis of rat bone marrow stromal cells (rBMSCs) compared to a positive control of scaffolds with encapsulated bone-morphogenic protein-2 (BMP-2). Poly(d,l-lactic-co-glycolic acid) (PLGA) microsphere-based scaffolds encapsulating TCP and HAp mixtures in two different ratios (7:3 and 1:1) were fabricated with the same net ceramic content (30 wt%) to evaluate how incorporation of these ceramic mixtures would affect the osteogenesis in rBMSCs. Encapsulation of TCP/HAp mixtures impacted microsphere morphologies and the compressive moduli of the scaffolds. Additionally, TCP/HAp mixtures enhanced the end-point secretion of extracellular matrix components relevant to bone tissue compared to the “blank” (PLGA-only) microsphere-based scaffolds as evidenced by the biochemical, gene expression, histology, and immunohistochemical characterization. Moreover, the TCP/HAp mixture groups even surpassed the BMP-2 positive control group in some instances in terms of matrix synthesis and gene expression. Lastly, gene expression data suggested that the rBMSCs responded differently to different TCP/HAp ratios presented to them. Altogether, it can be concluded that TCP/HAp mixtures stimulated the differentiation of rBMSCs toward an osteoblastic phenotype, and therefore may be beneficial in gradient microsphere-based scaffolds for osteochondral regeneration.












Similar content being viewed by others
References
Di Luca A, Van Blitterswijk C, Moroni L. The osteochondral interface as a gradient tissue: from development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res C. 2015;105(1):34–52.
Jeon JE, Vaquette C, Klein TJ, Hutmacher DW. Perspectives in multiphasic osteochondral tissue engineering. Anat Rec. 2013;297(1):26–35. doi:10.1002/ar.22795.
Lopa S, Madry H. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng A. 2014;20(15–16):2052–76. doi:10.1089/ten.tea.2013.0356.
Renth AN, Detamore MS. Leveraging “raw materials” as building blocks and bioactive signals in regenerative medicine. Tissue Eng B. 2012;18(5):341–62. doi:10.1089/ten.teb.2012.0080.
Seo SJ, Mahapatra C, Singh RK, Knowles JC, Kim HW. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng. 2014;5:1–14. doi:10.1177/2041731414541850.
Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng B. 2014;20:468–76. doi:10.1089/ten.teb.2013.0543.
GĂśtz W, Lenz S, Reichert C, Henkel K, Pernicka L, Gundlach K, et al. A preliminary study in osteoinduction by a nano-crystalline hydroxyapatite in the mini pig. Folia Histochem Cytobiol. 2011;48(4):589–98.
Lü X, Wang J, Li B, Zhang Z, Zhao L. Gene expression profile study on osteoinductive effect of natural hydroxyapatite. J Biomed Mater Res A. 2014;102(8):2833–41.
Fielding G, Bose S. SiO 2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013;9(11):9137–48.
Sungjin C. Implantation of tetrapod-shaped granular artificial bones or β-tricalcium phosphate granules in a canine large bone-defect model. J Vet Med Sci. 2014;76(2):229–35.
Bloemers FW, Blokhuis TJ, Patka P, Bakker FC, Wippermann BW, Haarman HJTM. Autologous bone versus calcium-phosphate ceramics in treatment of experimental bone defects. J Biomed Mater Res. 2003;66(2):526–31.
Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29(9):1177–88.
Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AM, de Ruiter A, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci. 2010;107(31):13614–9.
Huang W, Li X, Shi X, Lai C. Microsphere based scaffolds for bone regenerative applications. Biomater Sci. 2014;2(9):1145–53.
Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73(1):59–74.
Blaker JJ, Knowles JC, Day RM. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008;4(2):264–72. doi:10.1016/j.actbio.2007.09.011.
Borden M, Attawia M, Laurencin CT. The sintered microsphere matrix for bone tissue engineering: in vitro osteoconductivity studies. J Biomed Mater Res. 2002;61(3):421–9. doi:10.1002/jbm.10201.
Brown JL, Nair LS, Laurencin CT. Solvent/non-solvent sintering: a novel route to create porous microsphere scaffolds for tissue regeneration. J Biomed Mater Res B. 2008;86(2):396–406. doi:10.1002/jbm.b.31033.
Brown LR, Gombotz WR, Healy MS, inventors; US Patent Office, assignee. Very low temperature casting of controlled release microspheres1991.
Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, Hindenlang MD, et al. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules. 2008;9(7):1818–25. doi:10.1021/bm800031t.
Dormer NH, Busaidy K, Berkland CJ, Detamore MS. Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. J Oral Maxillofac Surg. 2011;69(6):e50–7.
Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38(6):2167–82.
Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100(1):162–70.
Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng A. 2011;17(21–22):2845–55. doi:10.1089/ten.tea.2011.0135.
Singh M, Dormer N, Salash JR, Christian JM, Moore DS, Berkland C, et al. Three-dimensional macroscopic scaffolds with a gradient in stiffness for functional regeneration of interfacial tissues. J Biomed Mater Res A. 2010;94(3):870–6.
Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng C. 2008;14(4):299–309. doi:10.1089/ten.tec.2008.0167.
Gupta V, Mohan N, Berkland CJ, Detamore MS. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Front Bioeng Biotechnol. 2015;3:1–15. doi:10.3389/fbioe.2015.00096.
Mohan N, Gupta V, Sridharan BP, Mellott AJ, Easley JT, Palmer RH, et al. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regen Med. 2015;10:709–28.
Chen Y, Wang J, Zhu XD, Tang ZR, Yang X, Tan YF, et al. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: in vitro and in vivo evidence. Acta Biomater. 2015;11:435–48. doi:10.1016/j.actbio.2014.09.028.
Ebrahimi M, Pripatnanont P, Suttapreyasri S, Monmaturapoj N. In vitrobiocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J Biomed Mater Res. 2013;102(1):52–61. doi:10.1002/jbm.b.32979.
Imranul Alam M, Asahina I, Ohmamiuda K, Takahashi K, Yokota S, Enomoto S. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials. 2001;22(12):1643–51. doi:10.1016/S0142-9612(00)00322-7.
Bhamidipati M, Sridharan B, Scurto AM, Detamore MS. Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering. Mater Sci Eng C. 2013;33(8):4892–9. doi:10.1016/j.msec.2013.08.010.
Dormer NH, Qiu Y, Lydick AM, Allen ND, Mohan N, Berkland CJ, et al. Osteogenic differentiation of human bone marrow stromal cells in hydroxyapatite-loaded microsphere-based scaffolds. Tissue Eng A. 2011;18(7–8):757–67.
Jeon JH, Bhamidipati M, Sridharan B, Scurto AM, Berkland CJ, Detamore MS. Tailoring of processing parameters for sintering microsphere-based scaffolds with dense-phase carbon dioxide. J Biomed Mater Res B. 2013;101(2):330–7. doi:10.1002/jbm.b.32843.
Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnol Bioeng. 2014;111(4):829–41.
Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS. Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent. Acta Biomater. 2010;6(1):137–43. doi:10.1016/j.actbio.2009.07.042.
Sridharan B, Mohan N, Berkland CJ, Detamore MS. Material characterization of microsphere-based scaffolds with encapsulated raw materials. Mater Sci Eng C. 2016;63:422–8.
Sutherland AJ, Detamore MS. Bioactive Microsphere-based scaffolds containing decellularized cartilage. Macromol Biosci. 2015;15(7):979–89.
Singh M, Detamore. Stress relaxation behavior of mandibular condylar cartilage under high-strain compression. J Biomech Eng. 2009;131(6):061008. doi:10.1115/1.3118776.
Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125(4):558. doi:10.1115/1.1589778.
Ghanaati S, Barbeck M, Detsch R, Deisinger U, Hilbig U, Rausch V, et al. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed Mater (Bristol, England). 2012;7(1):015005. doi:10.1088/1748-6041/7/1/015005.
Hu J, Zhou Y, Huang L, Liu J, Lu H. Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet Disord. 2014;15(1):114. doi:10.1186/1471-2474-15-114.
Kurashina K, Kurita H, Wu Q, Ohtsuka A, Kobayashi H. Ectopic osteogenesis with biphasic ceramics of hydroxyapatite and tricalcium phosphate in rabbits. Biomaterials. 2002;23(2):407–12. doi:10.1016/S0142-9612(01)00119-3.
Sanda M, Shiota M, Fujii M, Kon K, Fujimori T, Kasugai S. Capability of new bone formation with a mixture of hydroxyapatite and beta-tricalcium phosphate granules. Clin Oral Implant Res. 2014;26(12):1369–74. doi:10.1111/clr.12473.
Schopper C, Ziya-Ghazvini F, Goriwoda W, Moser D, Wanschitz F, Spassova E, et al. HA/TCP compounding of a porous CaP biomaterial improves bone formation and scaffold degradation—a long-term histological study. J Biomed Mater Res B. 2005;74(1):458–67. doi:10.1002/jbm.b.30199.
Wongwitwichot P, Kaewsrichan J, Chua K, Ruszymah B. Comparison of TCP and TCP/HA hybrid scaffolds for osteoconductive activity. Open Biomed Eng J. 2010;4:279–85.
Bao T-Q, Franco R-A, Lee B-T. Preparation and characterization of novel poly(ε-caprolactone)/biphasic calcium phosphate hybrid composite microspheres. J Biomed Mater Res. 2011;98B(2):272–9. doi:10.1002/jbm.b.31849.
Newbury DE. Mistakes encountered during automatic peak identification of minor and trace constituents in electron-excited energy dispersive X-ray microanalysis. Scanning. 2009;31(3):91–101.
Keaveny TM, Hayes WC. Mechanical properties of cortical and trabecular bone. Bone A. 1993;7:285–344.
Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A(11):2111–20.
Moroni L, de Wijn JR, van Blitterswijk CA. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27(7):974–85. doi:10.1016/j.biomaterials.2005.07.023.
Sudarmadji N, Tan JY, Leong KF, Chua CK, Loh YT. Investigation of the mechanical properties and porosity relationships in selective laser-sintered polyhedral for functionally graded scaffolds. Acta Biomater. 2011;7(2):530–7. doi:10.1016/j.actbio.2010.09.024.
Kucharska M, Walenko K, Lewandowska-Szumieł M, Brynk T, Jaroszewicz J, Ciach T. Chitosan and composite microsphere-based scaffold for bone tissue engineering: evaluation of tricalcium phosphate content influence on physical and biological properties. J Mater Sci Mater Med. 2015;26(3):143. doi:10.1007/s10856-015-5464-9.
Arinzeh TL, Tran T, Mcalary J, Daculsi G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 2005;26(17):3631–8. doi:10.1016/j.biomaterials.2004.09.035.
Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38(2):105–14. doi:10.1002/(SICI)1097-4636(199722)38:2<105:AID-JBM4>3.0.CO;2-U/asset/4_ftp.pdf.
Niemelä T. Effect of β-tricalcium phosphate addition on the in vitro degradation of self-reinforced poly-l, d-lactide. Polym Degrad Stab. 2005;89(3):492–500. doi:10.1016/j.polymdegradstab.2005.02.003.
Yang F, Cui W, Xiong Z, Liu L, Bei J, Wang S. Poly(l, l-lactide-co-glycolide)/tricalcium phosphate composite scaffold and its various changes during degradation in vitro. Polym Degrad Stab. 2006;91(12):3065–73. doi:10.1016/j.polymdegradstab.2006.08.008.
Acknowledgments
This publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01 AR056347). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to recognize support from the Kansas Bioscience Authority Rising Star Award. In addition, we thank Prem Thapa for his assistance with SEM imaging and EDS mapping.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, V., Lyne, D.V., Barragan, M. et al. Microsphere-based scaffolds encapsulating tricalcium phosphate and hydroxyapatite for bone regeneration. J Mater Sci: Mater Med 27, 121 (2016). https://doi.org/10.1007/s10856-016-5734-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5734-1