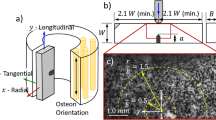
Abstract
As a composite material, cortical bone accumulates fatigue microdamage through the repetitive loading of everyday activity (e.g. walking). The accumulation of fatigue microdamage is thought to contribute to the occurrence of fragility fractures in older people. Therefore it is beneficial to understand the relationship between microcrack accumulation and the fracture resistance of cortical bone. Twenty longitudinally orientated compact tension fracture specimens were machined from a single bovine femur, ten specimens were assigned to both the control and fatigue damaged groups. The damaged group underwent a fatigue loading protocol to induce microdamage which was assessed via fluorescent microscopy. Following fatigue loading, non-linear fracture resistance tests were undertaken on both the control and damaged groups using the J-integral method. The interaction of the crack path with the fatigue induced damage and inherent toughening mechanisms were then observed using fluorescent microscopy. The results of this study show that fatigue induced damage reduces the initiation toughness of cortical bone and the growth toughness within the damage zone by three distinct mechanisms of fatigue–fracture interaction. Further analysis of the J-integral fracture resistance showed both the elastic and plastic component were reduced in the damaged group. For the elastic component this was attributed to a decreased number of ligament bridges in the crack wake while for the plastic component this was attributed to the presence of pre-existing fatigue microcracks preventing energy absorption by the formation of new microcracks.







Similar content being viewed by others
References
Burr DB, Forwood MR, Fyhrie DP, et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15.
Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone. 2006;38:427–31.
Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–5.
Zioupos P, Currey J. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone. 1998;22:57–66.
Waldorff EI, Goldstein SA, McCreadie BR. Age-dependent microdamage removal following mechanically induced microdamage in trabecular bone in vivo. Bone. 2007;40:425–32.
Danova N, Colopy S, Radtke C, et al. Degradation of bone structural properties by accumulation and coalescence of microcracks. Bone. 2003;33:197–205.
Zioupos P. Accumulation of in vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc. 2001;201:270–8.
Rho J-Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102.
Boyce TM, Fyhrie DP, Glotkowski MC, et al. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res. 1998;16:322–9.
Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37:96–102.
Koester KJ, Ager JW, Ritchie RO. The true toughness of human cortical bone measured with realistically short cracks. Nat Mater. 2008;7:672.
Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Effect of aging on the toughness of human cortical bone: evaluation by R-curves. Bone. 2004;35:1240–6.
Ritchie RO, Buehler MJ, Hansma P. Plasticity and toughness in bone. Phys Today. 2009;62:41–7.
Vashishth D, Tanner K, Bonfield W. Experimental validation of a microcracking-based toughening mechanism for cortical bone. J Biomech. 2003;36:121–4.
Zimmermann EA, Schaible E, Bale H, et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci USA. 2011;108:14416–21.
Norman TL, Yeni YN, Brown CU, Wang Z. Influence of microdamage on fracture toughness of the human femur and tibia. Bone. 1998;23:303–6.
Wang X, Puram S. The toughness of cortical bone and its relationship with age. Ann Biomed Eng. 2004;32:123–35.
Launey ME, Buehler MJ, Ritchie RO. On the mechanistic origins of toughness in bone. Annu Rev Mater Res. 2010;40:25–53.
Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech. 1997;30:763–9.
Paschalis EP, Shane E, Lyritis G, et al. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–4.
Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5:62–6.
Wang X, Shen X, Li X, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7.
Martin RB, Gibson VA, Stover SM, et al. Residual strength of equine bone is not reduced by intense fatigue loading: implications for stress fracture. J Biomech. 1997;30:109–14.
Parsamian GP, Norman TL. Diffuse damage accumulation in the fracture process zone of human cortical bone specimens and its influence on fracture toughness. J Mater Sci Mater Med. 2001;12:779–83.
Yeni Y, Fyhrie D. Fatigue damage-fracture mechanics interaction in cortical bone. Bone. 2002;30:509–14.
Barth HD, Launey ME, MacDowell AA, et al. On the effect of X-ray irradiation on the deformation and fracture behaviour of human cortical bone. Bone. 2010;46:1475–85.
Koester KJ, Barth HD, Ritchie RO. Effect of aging on the transverse toughness of human cortical bone: evaluation by R-curves. J Mech Behav Biomed Mater. 2011;4:1504–13.
Kulin RM, Jiang F, Vecchio KS. Loading rate effects on the R-curve behaviour of cortical bone. Acta Biomater. 2011;7:724–32.
Nalla R, Kruzic J, Kinney J, Ritchie R. Mechanistic aspects of fracture and R-curve behaviour in human cortical bone. Biomaterials. 2005;26:217–31.
Vashishth D. Rising crack-growth-resistance behaviour in cortical bone: implications for toughness measurements. J Biomech. 2004;37:943–6.
Yan J, Mecholsky JJ Jr, Clifton KB. How tough is bone? Application of elastic–plastic fracture mechanics to bone. Bone. 2007;40:479–84.
Yang QD, Cox BN, Nalla RK, Ritchie RO. Fracture length scales in human cortical bone: the necessity of nonlinear fracture models. Biomaterials. 2006;27:2095–113.
Yan J, Taskonak B, Platt JA, Mecholsky JJ Jr. Evaluation of fracture toughness of human dentin using elastic–plastic fracture mechanics. J Biomech. 2008;41:1253–9.
ASTM Standard E1820. Test method for measurement of fracture toughness. West Conshohocken: ASTM International; 2011.
Fletcher L, Codrington J, Parkinson I. Effects of irradiation and non-enzymatic glycation on the fracture resistance of bovine cortical bone. In: Proc 7th Australas Congr Appl Mech ACAM 7 9-12 Dec 2012 Univ Adel North Terrace Campus Natl Comm Appl Mech Eng Aust 322. 2012
Reilly DT, Burstein AH. The elastic and ultimate properties of compact bone tissue. J Biomech. 1975;8:393–405.
Nalla RK, Kruzic JJ, Ritchie RO. On the origin of the toughness of mineralized tissue: microcracking or crack bridging? Bone. 2004;34:790–8.
O’Brien FJ, Taylor D, Clive Lee T. The effect of bone microstructure on the initiation and growth of microcracks. J Orthop Res. 2005;23:475–80.
Fleck C, Eifler D. Deformation behaviour and damage accumulation of cortical bone specimens from the equine tibia under cyclic loading. J Biomech. 2003;36:179–89.
O’Brien FJ, Taylor D, Clive Lee T. Bone as a composite material: the role of osteons as barriers to crack growth in compact bone. Int J Fatigue. 2007;29:1051–6.
Lipson SF, Katz JL. The relationship between elastic properties and microstructure of bovine cortical bone. J Biomech. 1984;17:231–40.
Acknowledgments
In kind support for this research was provided by the Bone and Joint Research Laboratory, SA Pathology and the School of Medical Science, The University of Adelaide.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fletcher, L., Codrington, J. & Parkinson, I. Effects of fatigue induced damage on the longitudinal fracture resistance of cortical bone. J Mater Sci: Mater Med 25, 1661–1670 (2014). https://doi.org/10.1007/s10856-014-5213-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5213-5