Abstract
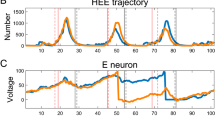
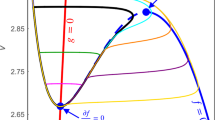
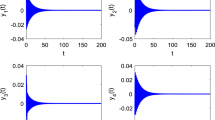
We present a numerical analysis of the dynamics of all-to-all coupled Hodgkin-Huxley (HH) neuronal networks with Poisson spike inputs. It is important to point out that, since the dynamical vector of the system contains discontinuous variables, we propose a so-called pseudo-Lyapunov exponent adapted from the classical definition using only continuous dynamical variables, and apply it in our numerical investigation. The numerical results of the largest Lyapunov exponent using this new definition are consistent with the dynamical regimes of the network. Three typical dynamical regimes—asynchronous, chaotic and synchronous, are found as the synaptic coupling strength increases from weak to strong. We use the pseudo-Lyapunov exponent and the power spectrum analysis of voltage traces to characterize the types of the network behavior. In the nonchaotic (asynchronous or synchronous) dynamical regimes, i.e., the weak or strong coupling limits, the pseudo-Lyapunov exponent is negative and there is a good numerical convergence of the solution in the trajectory-wise sense by using our numerical methods. Consequently, in these regimes the evolution of neuronal networks is reliable. For the chaotic dynamical regime with an intermediate strong coupling, the pseudo-Lyapunov exponent is positive, and there is no numerical convergence of the solution and only statistical quantifications of the numerical results are reliable. Finally, we present numerical evidence that the value of pseudo-Lyapunov exponent coincides with that of the standard Lyapunov exponent for systems we have been able to examine.

















Similar content being viewed by others
References
Aihara, K., & Matsumoto, G. (1986). Chaotic oscillations and bifurcations in squid giant axons. In Chaos, nonlinear science: Theory and applications. Manchester: Manchester University Press.
Cai, D., Rangan, A. V., & McLaughlin, D. W. (2005). Architectural and synaptic mechanisms underlying coherent spontaneous activity in v1. Proceedings of the National Academy of Sciences (USA), 102, 5868–5873.
Campbell, D., & Rose, H. (Eds.) (1983). Order in chaos. Physica D, 7, 1–362.
Compte, A., Sanchez-Vives, M. V., McCormick, D. A., & Wang, X. J. (2003). Cellular and network mechanisms of slow oscillatory activity (< 1 Hz) and wave propagations in a cortical network model. Journal of Neurophysiology, 89, 2707–2725.
Dayan, P., & Abbott, L. F. (2001). Theoretical neuroscience: Computational and mathematical modeling of neural systems. Cambridge: MIT.
Guckenheimer, J., & Oliva, R. A. (2002). Chaos in the Hodgkin-Huxley model. SIAM Journal on Applied Dynamical Systems, 1, 105–114.
Hansel, D., Mato, G., Meunier, C., & Neltner, L. (1998). On numerical simulations of integrate-and-fire neural networks. Neural Computation, 10, 467–483.
Hansel, D., & Sompolinsky, H. (1992). Synchronization and computation in a chaotic neural network. Physical Review Letters, 68, 718–721.
Hansel, D., & Sompolinsky, H. (1996). Chaos and synchrony in a model of a hypercolumn in visual cortex. Journal of Computational Neuroscience, 3, 7–34.
Hodgkin, A. L., & Huxley, A. F. (1952). A. quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Koch, C. (1999). Biophysics of computation. Oxford: Oxford University Press.
Kosmidis, E. K., & Pakdaman, K. (2003). An analysis of the reliability phenomenon in the FitzHugh-Nagumo model. Journal of Computational Neuroscience, 14, 5–22.
Lin, K. (2006). Entrainment and chaos in a pulse-driven Hodgkin-Huxley oscillator. SIAM Journal on Applied Dynamical Systems, 5, 179–204.
Mainen, Z., & Sejnowski, T. (1995). Reliability of spike timing in neocortical neurons. Science, 268, 1503–1506.
Mattia, M., & Del Giudice, P. (2000). Efficient event-driven simulation of large networks of spiking neurons and dynamical synapses. Neural Computation, 12, 2305–2329.
McLaughlin, D., Shapley, R., Shelley, M., & Wielaard, J. (2000). A neuronal network model of macaque primary visual cortex (V1): Orientation selectivity and dynamics in the input layer 4Cα. Proceedings of the National Academy of Sciences USA, 97, 8087–8092.
Mueller, P. (1995). Calculation of Lyapunov exponents for dynamic systems with discontinuities. Chaos, Solitons & Fractals, 5, 1671–1681.
Ott, E. (1993). Chaos in dynamical systems. New York: Cambridge University Press.
Parker, T. S., & Chua, L. O. (1989). Practical numerical algorithms for chaotic systems. New York: Springer.
Rangan, A. V., & Cai, D. (2007). Fast numerical methods for simulating large-scale integrate-and-fire neuronal networks. Journal of Computational Neuroscience, 22, 81–100.
Rangan, A. V., Cai, D., & McLaughlin, D. W. (2005). Modeling the spatiotemporal cortical activity associated with the line-motion illusion in primary visual cortex. Proceedings of the National Academy of Sciences (USA), 102, 18793–18800.
Reutimann, J., Giugliano, M., & Fusi, S. (2003). Event-based simulation of spiking neurons with stochastic dynamics. Neural Computation, 15, 811–830.
Rudolph, M., & Destexhe, A. (2007). How much can we trust neural simulation strategies? Neurocomputing, 70, 1966–1969.
Schuster, H. G., & Just, W. (2005). Deterministic chaos. Weinheim: Wiley-VCH Verlag.
Shelley, M. J., & Tao, L. (2001). Efficient and accurate time-stepping schemes for integrate-and-fire neuronal networks. Journal of Computational Neuroscience, 11, 111–119.
Somers, D., Nelson, S., & Sur, M. (1995). An emergent model of orientation selectivity in cat visual cortical simple cells. Journal of Neuroscience, 15, 5448–5465.
Sun, Y., Zhou, D., Rangan, A. V., & Cai, D. (2009). Library-based numerical reduction of the Hodgkin-Huxley neuron for network simulation. Journal of Computational Neuroscience, 27, 369–390.
Takens, F. (1981). Detecting strange attractors in turbulence. In Dynamical systems and turbulence, lecture notes in mathematics (Vol. 898). Berlin: Springer.
Troyer, T., Krukowski, A., Priebe N., & Miller, K. (1998). Contrast invariant orientation tuning in cat visual cortex with feedforward tuning and correlation based intracortical connectivity. Journal of Neuroscience, 18, 5908–5927.
Acknowledgements
The work was supported by NSF grants DMS-0506396, DMS-0507901 and a grant from the Swartz foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Terman
Appendices
Parameter values for the Hodgkin-Huxley equations
Parameter values or ranges and function definitions of the Hodgkin-Huxley model are as follows (Dayan and Abbott 2001):
Numerical method for a single neuron
Here we provide details of the numerical method for evolving the dynamics of a single neuron. For simplicity, we use vector X i to represent all the variables in the solution of the ith neuron:
Given an initial time t 0 and time step Δt, initial values X i (t 0), and spike times \(T^{\rm F}_{i,k}\) and \(T^{\rm S}_{j\neq i,k}\) from the rest of the network, our method computes a numerical solution of all variables X i (t 0 + Δt) as well as the intervening spike times \(T^{\rm S}_{i,k}\) (if any occurred) for the ith neuron as follows:
Algorithm 1. (Single neuron scheme)
Step 1: Input: an initial time t0, a time step Δt, a set of spike times \(T^{\rm F}_{i,k}\) and \(T^{\rm S}_{j\neq i,k}\) and associated strengths \(F_i^{\rm Q}\) and \(S_{i,j}^{\rm Q}\).
Step 2: Consider the time interval [t0, t0 + Δt]. Let M denote the total number of feedforward and presynaptic spikes within this interval. Sort these spikes into an increasing list of M spike times \(T^{\rm sorted}_m\) with corresponding spike strengths \(S^{\rm sorted, Q}_m\). In addition, we extend this notation such that \(T^{\rm sorted}_0\) : = t0, \(T^{\rm sorted}_{M+1}:= t_0 + \Delta t\) and \(S^{\rm sorted, Q}_0 =S^{\rm sorted, Q}_{M+1}:= 0\).
Step 3: For m = 1, ..., M + 1, advance the equations for the HH neuron model and its conductances (Eqs. (1)–(7)) from \(T^{\rm sorted}_{m-1}\) to \(T^{\rm sorted}_m\) using the standard RK4 scheme to obtain \(X_i(T^{\rm sorted}_m)\); Then, update the conductance \(\widetilde{G}_i^{\rm Q}(T^{\rm sorted}_m)\) by adding the appropriate strengths \(S^{\rm sorted, Q}_m\).
Step 4: If the calculated values for \(V_i(T^{\rm sorted}_m)\) are each less than Vth, then we can accept \(X_i(T^{\rm sorted}_{M+1})\) as the solution X i (t0 + Δt). We update t0 ←t0 + Δt and return to step 2 and continue.
Step 5: Otherwise, let \(V_i(T^{\rm sorted}_m)\) be the first calculated voltage greater than Vth. We know that the ith neuron fired somewhere during the interval \([T^{\rm sorted}_{m-1}, T^{\rm sorted}_m]\).
Step 6: In this case we use a high-order polynomial interpolation to find an approximation of the spike time tfire in the interval \([T^{\rm sorted}_{m-1}, T^{\rm sorted}_m]\). For example, we can use the numerical values of \(V_i(T^{\rm sorted}_{m-1})\), \(V_i(T^{\rm sorted}_m)\), \(\frac{d}{dt}V_i(T^{\rm sorted}_{m-1})\), \(\frac{d}{dt}V_i(T^{\rm sorted}_m)\) to form a cubic polynomial. We record tfire as the (k + 1)th postsynaptic spike time \(T^{\rm S}_{i,k+1}\) of the ith neuron. We update t0 ←t0 + Δt and return to step 2 and continue.
Numerical method for the HH network
Here we briefly outline an algorithm which accounts for spike-spike interactions, and can accurately evolve recurrent HH networks governed by Eqs. (1)–(7).
Algorithm 2. (Network model scheme)
Step 1: Given initial values initial values X i (t0) and feedforward input spike times \(T^{\rm F}_{i,k}\) for all i, we can apply Algorithm 1 and time step Δt to obtain an estimate of neuronal trajectories on the interval [t0, t0 + Δt].
Step 2: We use this rough estimate to classify the neurons into two groups  ,
,  —those that are estimated to fire within [t0, t0 + Δt] and those that are not.
—those that are estimated to fire within [t0, t0 + Δt] and those that are not.
Step 3: We sort the approximate spike times of neurons within  into a list
into a list  with corresponding coupling strengths
with corresponding coupling strengths  .
.
Step 4: We use the feedforward spikes \(T^{\rm F}_{i,k}\) as well as the approximate spike times  and Algorithm 1 and the same time step Δt to correct the neuronal trajectories of the subnetwork
and Algorithm 1 and the same time step Δt to correct the neuronal trajectories of the subnetwork  over the interval [t0, t0 + Δt], and obtain a more accurate approximation to the spike times
over the interval [t0, t0 + Δt], and obtain a more accurate approximation to the spike times  . This spike-spike correction procedure is equivalent to stepping through the list
. This spike-spike correction procedure is equivalent to stepping through the list  and computing the effect of each spike on all future spikes. We step through this correction process until the neuronal trajectories and spike times of neurons in
and computing the effect of each spike on all future spikes. We step through this correction process until the neuronal trajectories and spike times of neurons in  converge.
converge.
Step 5: Finally we use the corrected estimates of the spike times  as well as the feedforward spikes \(T^{\rm F}_{i,k}\) and Algorithm 1 and time step Δt to evolve the remainder of the neurons
as well as the feedforward spikes \(T^{\rm F}_{i,k}\) and Algorithm 1 and time step Δt to evolve the remainder of the neurons  from t0 to t0 + Δt.
from t0 to t0 + Δt.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhou, D., Rangan, A.V. et al. Pseudo-Lyapunov exponents and predictability of Hodgkin-Huxley neuronal network dynamics. J Comput Neurosci 28, 247–266 (2010). https://doi.org/10.1007/s10827-009-0202-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0202-2