Abstract
Purpose
The main goals of this study were to investigate the expression of anti-Müllerian hormone (AMH) and its receptor (AMHR2) during follicular development in primates, and to evaluate the potential of AMH as a biomarker for follicle growth and oocyte maturation in vitro.
Methods
The mRNA and protein expression of AMH and AMHR2 were determined using isolated follicles and ovarian sections from rhesus macaques (n = 4) by real-time PCR and immunohistochemistry, respectively. Isolated secondary follicles were cultured individually. Follicle growth and media AMH concentrations were assessed by ELISA. The mRNA expression profiles, obtained from RNA sequencing, of in vitro- and in vivo-developed antral follicles were compared. Secondary follicles from additional animals (n = 35) were cultured. Follicle growth, oocyte maturation, and media AMH concentrations were evaluated for forecasting follicular development in vitro by AMH levels.
Results
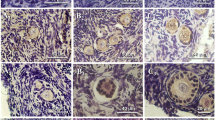
AMH immunostaining was heterogeneous in the population of preantral follicles that were also stained for AMHR2. The mRNA expression profiles were comparable between in vivo- and in vitro-developed follicles. AMH levels produced by growing follicles were higher than those of nongrowing follicles in culture. With a cutoff value of 1.40 ng/ml, 85 % of nongrowing follicles could be identified while eliminating only 5 % of growing follicles. Growing follicles that generated metaphase II-stage oocytes secreted greater amounts of AMH than did those yielding immature germinal vesicle-stage oocytes.
Conclusions
AMH, co-expressed with AMHR2, was produced heterogeneously by preantral follicles in macaques with levels correlated positively with follicle growth and oocyte maturation. AMH may serve as a biomarker for primate follicular development in vitro.




Similar content being viewed by others
References
Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601–9.
Rico C, Médigue C, Fabre S, Jarrier P, Bontoux M, Clément F, et al. Regulation of anti-Müllerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod. 2011;84:560–71.
Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–7.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85.
Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct. Development. 1994;120:189–97.
Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–62.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–9.
Thomas FH, Telfer EE, Fraser HM. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–81.
Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–200.
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83.
Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during 3-dimensional culture. Hum Reprod. 2015;30:664–74.
Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94.
Xu F, Stouffer RL, Müller J, Hennebold JD, Wright JW, Bahar A, et al. Dynamics of the 604 transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–65.
Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod. 2010;83:525–32.
Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–72.
Xu J, McGee WK, Bishop CV, Park BS, Cameron JL, Zelinski MB, et al. Exposure of female macaques to Western-style diet with or without chronic T in vivo alters secondary follicle function during encapsulated 3-dimensional culture. Endocrinology. 2015;156:1133–42.
Lohse M, Bolger AM, Nagel A, Fernie AR, Lunn JE, Stitt M, et al. RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012;40(Web Server issue):W622–7.
Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–81.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50.
Pavlov IY, Wilson AR, Delgado JC. Reference interval computation: which method (not) to choose? Clin Chim Acta. 2012;413:1107–14.
Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–97.
Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 2003;211:65–73.
Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes preantral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31:1522–30.
Ting AY, Xu J, Stouffer RL. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod. 2015;30:1907–17.
Skory RM, Bernabé BP, Galdones E, Broadbelt LJ, Shea LD, Woodruff TK. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev. 2013;80:132–44.
Sánchez F, Adriaenssens T, Romero S, Smitz J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol Hum Reprod. 2009;15:539–50.
McNatty KP, Makris A, Osathanondh R, Ryan KJ. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids. 1980;36:53–63.
Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46.
Stouffer RL, Martínez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001;32:567–75.
Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–7.
Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am J Physiol Endocrinol Metab. 2014;306:E893–903.
Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:d222–37.
Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11.
Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323.
Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206.
Acknowledgments
We are thankful for the assistance provided by members of the Division of Comparative Medicine, the Endocrine Technology Support Core, the Histopathology-Morphology Research Core, the Assisted Reproductive Technologies Support Core, the Molecular & Cellular Biology Support Core, and the Biostatistics and Bioinformatics Unit at ONPRC, as well as the OHSU Massively Parallel Sequencing Shared Resource. The valuable assistance of Ms. Maralee Lawson at ONPRC with follicle culture is appreciated. We are grateful to Dr. Richard L. Stouffer at ONPRC for his valuable expertise and critical review of the manuscript.
Research reported in this publication was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082208, NIH Office of Research on Women’s Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women’s Health, BIRCWH), NIH Office of the Director P51OD011092 ONPRC Pilot Grant, Collins Medical Trust, American Society for Reproductive Medicine, and Medical Research Foundation of Oregon. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082208, NIH Office of Research on Women’s Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women’s Health, BIRCWH), NIH Office of the Director P51OD011092 ONPRC Pilot Grant, Collins Medical Trust, American Society for Reproductive Medicine, and Medical Research Foundation of Oregon.
Additional information
Capsule
AMH, co-expressed with AMHR2, was produced heterogeneously by preantral follicles in macaques with levels correlated positively with follicle growth and oocyte maturation. AMH may serve as a biomarker for primate follicular development in vitro.
Rights and permissions
About this article
Cite this article
Xu, J., Xu, F., Letaw, J.H. et al. Anti-Müllerian hormone is produced heterogeneously in primate preantral follicles and is a potential biomarker for follicle growth and oocyte maturation in vitro. J Assist Reprod Genet 33, 1665–1675 (2016). https://doi.org/10.1007/s10815-016-0804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0804-3