Abstract
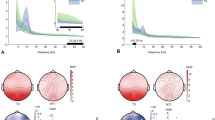
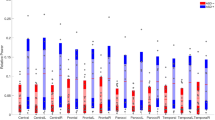
The electrophysiological correlates of cognitive deficits in tuberous sclerosis complex (TSC) are not well understood, and modulations of neural dynamics by neuroanatomical abnormalities that characterize the disorder remain elusive. Neural oscillations (rhythms) are a fundamental aspect of brain function, and have dominant frequencies in a wide frequency range. The spatio-temporal dynamics of these frequencies in TSC are currently unknown. Using a novel signal decomposition approach this study investigated dominant cortical frequencies in 10 infants with TSC, in the age range 18–30 months, and 12 age-matched healthy controls. Distinct spectral characteristics were estimated in the two groups. High-frequency [in the high-gamma (>50 Hz) and ripple (>80 Hz) ranges], non-random EEG components were identified in both TSC and healthy infants at 18 months. Additional components in the lower gamma (30–50 Hz) ranges were also identified, with higher characteristic frequencies in TSC than in controls. Lower frequencies were statistically identical in both sub-groups. A significant shift in the high-frequency spectral content of the EEG was observed as a function of age, independently of task performance, possibly reflecting an overall maturation of developing neural circuits. This shift occurred earlier in healthy infants than in TSC, i.e., by age 20 months the highest dominant frequencies were in the high gamma range, whereas in TSC dominant frequencies above 100 Hz were still measurable. At age 28–30 months a statistically significant decrease in dominant high frequencies was observed in both TSC and healthy infants, possibly reflecting increased myelination and neuronal connection strengthening with age. Although based on small samples, and thus preliminary, the findings in this study suggest that dominant cortical rhythms, a fundamental aspect of neurodynamics, may be affected in TSC, possibly leading to impaired information processing in the brain.













Similar content being viewed by others
References
Au, K. S., Williams, A. T., Roach, E. S., et al. (2007). Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genetics in Medicine, 9(2), 88–100.
Bolton, P. F., Park, R. J., Higgins, J. N., et al. (2002). Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain, 125(Pt6), 1247–1255.
Burgess, A. P. (2012). Towards a unified understanding of event-related changes in the EEG: The firefly model of synchronization through cross-frequency phase modulation. PLoS One, 7(9), e45630.
Crino, P. B., Nathanson, K. L., & Henske, E. P. (2006). The tuberous sclerosis complex. New England Journal of Medicine, 355, 1345–1356.
Dabora, S. L., Jozwiak, S., Franz, D. N., et al. (2001). Mutational analysis in a cohort of 224 tuberous sclerosis patients indicated increased severity of TSC2, compared with TSC1, disease in multiple organs. American Society of Human Genetics, 68(1), 64–80.
D’Argenzio, L., Koch, G., Bombardieri, R., et al. (2009). Abnormal parieto-motor connectivity in tuberous sclerosis complex. Epilepsy Research, 87(1), 102-5.
Deoni, S. C., Mercure, E., & Blasi, A. (2011). Mapping infant brain myelination with magnetic resonance imaging. Journal of Neuroscience, 31(2), 784–791.
EGI Technical Note (2005). Determination of the Geodesic Sensor Nets average electrode positions and their 10-10 international equivalents, Electrical Geodesics, Inc., Eugene, OR.
Gallagher, A., Chu-Shore, C. J., Montenegro, M. A., et al. (2009). Associations between electroencephalographic and magnetic resonance imaging findings in tuberous sclerosis complex. Epilepsy Research, 87(2-3), 197–202.
Geddes, L. A., & Baker, L. E. (1967). The specific resistance of biological material, a compendium of data for the biomedical engineer and physiologist. Medical and Biological Engineering and Computing, 5, 271–293.
Guttierez, G. C., Smalley, S. L., & Tanguay, P. E. (1998). Autism in tuberous sclerosis complex. Journal of Autism and Developmental Disorders, 28, 97–103.
Harrison, J. E., & Bolton, P. F. (1997). Annotation: Tuberous sclerosis. Journal of Child Psychology and Psychiatry, 38(6), 603–614.
Holmes, G. L., & Stafstrom C. E. (2007). Tuberous sclerosis study group, tuberous sclerosis complex and epilepsy: Recent developments and future challenges. Epilepsia, 48(4), 617–630.
Huang, N. E., Shen, Z., Long, S. R., et al. (1998). Empirical Mode Decomposition and Hilbert spectrum for non-linear, non-stationary time series analysis. Proceedings of the Royal Society of London, Series A: Mathematical and Physical Sciences, 454, 903–995.
Irahara, K., Nakagawa, E., Honda, R., et al. (2012). High gamma activity of 60–70 Hz in the area surrounding a cortical tuber in an infant with tuberous sclerosis. Italian Journal of Pediatrics, 38, 15.
Jacobs, J., Rohr, A., Moeller, F., et al. (2008). Evaluation of epileptogenic networks in children with tuberous sclerosis complex using EEG-fMRI. Epilepsia, 49(5), 816–825.
Jansen, F. E., Braams, O., Vincken, K. L., et al. (2008). Overlapping neurologic and cognitive phenotypes in patients with TSC1 and TSC2 mutations. Neurology 70(12), 908–915.
Jeste, S. S., Sahin, M., Bolton, P. et al. (2008). Characterization of autism in young children with tuberous sclerosis complex. Journal of Child Neurology, 23(5), 520–525.
Kandt, R. S., Haines, J. L., Smith, M. et al. (1992). Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease. Nature Genetics, 237–241.
Kleinhans, N. M., Richards, T., Sterling, L., et al. (2008). Abnormal functional connectivity in autism spectrum disorders during face processing. Brain, 131, 1000–1012.
Major, P., Rakowski, S., Simon, M. V., et al. (2009). Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia, 50(1), 147–54.
Markel, J. D. (1972). The SIFT algorithm for fundamental frequency estimation. IEEE Transactions on Audio and Electroacoustics, 20, 367–377.
McGrath, J., Johnson, K., Ecker, C., et al. (2012). Atypical visuospatial processing in autism: Insights from functional connectivity analysis. Autism Research, 5(5), 314–30.
Muzykewicz, D. A., Newberry, P., Danforth, N., et al. (2007). Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy and Behavior, 11, 506–513.
Nebel, M. B., Joel, S. E., Muschelli, J., et al. (2012). Disruption of functional organization within the primary motor cortex in children with autism, Human Brain Mapping (Epub ahead of press).
Numis, A. L., Major, P., Montenegro, M. A., et al. (2011). Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology, 76(11), 981–987.
Oostendorp, T. F., Delbecke, J., Stegeman, D. F. (2000). The conductivity of the human skull: Results of in vivo and in vitro measurements. IEEE Transactions on Biomedical Engineering, 47, 1487–1492.
Peters, J. M., Sahin, M., Vogel-Farley, V. K., et al. (2012). Loss of white matter micro-structural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Academic Radiology, 19(1), 17–25.
Phadke, A., Thorp, J., & Adamiak, M. (1983). A new measurement technique for tracking voltage phasors, local systems frequency, and rate of change of frequency. IEEE Transaction on Power Applications System, PAS-102, 1025–1038.
Shepherd, C. W., & Stephenson, J. B. P. (1992). Seizures and intellectual disability associated with tuberous sclerosis complex in the West of Scotland. Developmental Medicine and Child Neurology, 34, 766–774.
Stamoulis, C., & Chang, B. S. (2009). Application of matched-filtering to extract EEG features and decouple signal contributions from multiple seizure foci in brain malformations. In IEEE Proceedings of the 4th International IEEE/EBMS Conference on Neural Engineering, (pp. 514–517).
Stamoulis, C., & Betensky, R. A. (2011). A novel signal processing approach for the detection of copy number variations in the human genome. Bioinformatics, 27(17), 2338–2345.
Sweeney, K., McLoone, S., & Ward, T. (2012). The use of ensemble empirical mode decomposition with canonical correlation analysis as a novel artifact removal technique, IEEE Transactions on Biomedical Engineering (Epub ahead of print).
Sweeney-Reed, C. M., & Nasuto, S. J. (2009). Detection of neural correlates of self-paced motor activity using empirical model decomposition phase locking analysis. Journal of Neuroscience Methods, 184(1), 54–70.
Tsai, P. H., Lin, C., Tsao, J., et al. (2012). Empirical mode decomposition based detrended sample entropy in electroencephalography for Alzheimer’s disease. Journal of Neuroscience Methods, 210(2), 230–237.
Thomson, D. J. (1982). Spectrum estimation and harmonic analysis. Proceedings of the IEEE, 70, 1055–1096.
Van Slegtenhorst, M., De Hoogt, R., Hermans, C., et al. (1997). Identification of the Tuberous Sclerosis gene TSC1 on chromomosome 9q34. Science, 277(5327), 805–808.
Webb, D. W., Fryer, A. E., & Osborne, J. P. (1991). On the incidence of fits and mental retardation in tuberous sclerosis. Journal of Medical Genetics, 28, 395–397.
Wu, Z., & Huang, N. E. (2004). A study of the characteristics of white noise using the empirical model decomposition method. Proceedings of the Royal Society of London, 460, 1597–1611.
Acknowledgments
This study was supported in part by the department of Radiology, Boston Children’s Hospital (CS), the Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) (CS), and a Department of Defense grant (W81XWH-10) (CA). The content is solely the responsibility of the authors. We are grateful to the families of participating subjects, as they have made this research possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stamoulis, C., Vogel-Farley, V., Degregorio, G. et al. Resting and Task-Modulated High-Frequency Brain Rhythms Measured by Scalp Encephalography in Infants with Tuberous Sclerosis Complex. J Autism Dev Disord 45, 336–353 (2015). https://doi.org/10.1007/s10803-013-1887-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-013-1887-7