Abstract
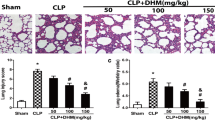
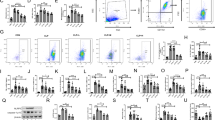
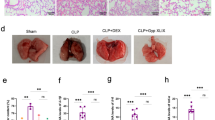
In current study, we investigated the role of liriodendrin, a constituent isolated from Sargentodoxa cuneata (Oliv.) Rehd. Et Wils (Sargentodoxaceae), in cecal ligation and puncture (CLP)-induced acute lung inflammatory response and injury (ALI). The inflammatory mediator levels in bronchoalveolar lavage fluid (BALF) were determined by enzyme-linked immunosorbent assay (ELISA). Pathologic changes in lung tissues were evaluated via pathological section with hematoxylin and eosin (H&E) staining. To investigate the mechanism whereby liriodendrin regulates lung inflammation, the phosphorylation of the NF-kB (p65) and expression of vascular endothelial growth factor (VEGF) were determined by western blot assay. We show that liriodendrin treatment significantly improved the survival rate of mice with CLP-induced sepsis. Pulmonary histopathologic changes, alveolar hemorrhage, and neutrophil infiltration were markedly decreased by liriodendrin. In addition, liriodendrin decreased the production of the proinflammatory mediators including (TNF-α, IL-1β, MCP-1, and IL-6) in lung tissues. Vascular permeability and lung myeloperoxidase (MPO) accumulation in the liriodendrin-treated mice were substantially reduced. Moreover, liriodendrin treatment significantly suppressed the expression of VEGF and activation of NF-kB in the lung. We further show that liriodendrin significantly reduced the production of proinflammatory mediators and downregulated NF-kB signaling in LPS-stimulated RAW 264.7 macrophage cells. Moreover, liriodendrin prevented the generation of reactive oxygen species (ROS) by upregulating the expression of SIRT1 in RAW 264.7 cells. These findings provide a novel theoretical basis for the possible application of liriodendrin in clinic.







Similar content being viewed by others
References
Lee, W.L., and A.S. Slutsky. 2010. Sepsis and endothelial permeability. New England Journal of Medicine 363: 689–691.
Marshall, J.C., J.L. Vincent, G. Guyatt, et al. 2005. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Critical Care Medicine 33: 1708–1716.
WJ, Fu lkerson., N. MacIntyre, J. Stamler, and J.D. Crapo. 1996. Pathogenesis and treatment of the adult respiratory distress syndrome. Archives of Internal Medicine 156: 29.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. New England Journal of Medicine 3(42): 1334.
Gao, H., T. Neff, and P.A. Ward. 2006. Regulation of lung inflammation in the model of IgG immune-complex injury. Annual Review of Pathology 1: 215–242.
Fan, J., R.D. Ye, and A.B. Malik. 2001. Transcriptional mechanisms of acute lung injury. American Journal of Physiology - Lung Cellular and Molecular Physiology 281: L1037–L1050.
Singer, D.R., S.J. Galli, A.M. Dvorak, C.A. Peruzzi, V.S. Harvey, and H.F. Dvorak. 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 17: 983–985.
Leung, D.W., G. Cachianes, W.J. Kuang, D.V. Goeddel, and N. Ferrara. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 17: 1306–1309.
Kuenen, B.C., M. Levi, J.C. Meijers, A.K. Kakkar, V.W. van Hinsbergh, P.J. Kostense, H.M. Pinedo, and K. Hoekman. 2002. Analysis of coagulation cascade and endothelial cell activation during inhibition of vascular endothelial growth factor/vascular endothelial growth factor receptor pathway in cancer patients. Arteriosclerosis, Thrombosis, and Vascular Biology 17: 1500–1505.
Maretta, M., S. Toth, Z. Jonecova, P. Kruzliak, P. Kubatka, S. Pingorova, and J. Vesela. 2014. Immunohistochemical expression of MPO, CD163 and VEGF in inflammatory cells in acute respiratory distress syndrome: a case report. International Journal of Clinical and Experimental Pathology 7(7): 4539–44.
Bosmann, M., N.F. Russkamp, B. Strobl, J. Roewe, L. Balouzian, F. Pache, M.P. Radsak, N. van Rooijen, F.S. Zetoune, J.V. Sarma, G. Núñez, M. Müller, P.J. Murray, and P.A. Ward. 2014. Interruption of macrophage-derived IL-27(p28) production by IL-10 during sepsis requires STAT3 but not SOCS3. Journal of Immunology 193(11): 5668–77.
Underhill, D.M., and A. Ozinsky. 2002. Phagocytosis of microbes: Complexity in action. Annual Review of Immunology 20: 825–852.
Jung, H.J., H.J. Park, R.G. Kim, K.M. Shin, J. Ha, J.W. Choi, et al. 2003. In vivo Anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus. Planta Medica 69: 610–616.
Feng, C., B.G. Li, X.P. Gao, H.Y. Qi, and G.L. Zhang. 2010. A new triterpene and an antiarrhythmic liriodendrin from Pittosporum brevicalyx. Archives of Pharmacal Research 33: 1927–1932.
Zhang, P., S.Q. Yan, Y.D. Shao, and Z.L. Li. 1988. Effect of some water soluble substances of Sargentodoxa cuneata on myocardial ischemia. Acta Academiae Medicinae Primae Shanghai 15: 191–194.
Lami, N., S. Kadota, T. Kikuchi, and Y. Momose. 1991. Constituents of the roots of Boerhaavia diffusa L. III. Identification of Ca2+ channel antagonistic compound from the methanol extract. Chemical & Pharmaceutical Bulletin 39: 1551–1555.
Ran, X.K., X.T. Wang, P.P. Liu, Y.X. Chi, B.J. Wang, D.Q. Dou, et al. 2013. Cytotoxic constituents from the leaves of Broussonetia papyrifera. Chinese Journal of Natural Medicines 11: 269–273.
Nam, J.W., S.Y. Kim, T. Yoon, Y.J. Lee, Y.S. Kil, Y.S. Lee, et al. 2013. Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents. Chemistry & Biodiversity 10: 1322–1327.
Sohn, Y.A., S.A. Hwang, S.Y. Lee, I.Y. Hwang, S.W. Kim, S.Y. Kim, A. Moon, Y.S. Lee, Y.H. Kim, K.J. Kang, and C.S. Jeong. 2015. Protective Effect of Liriodendrin Isolated from Kalopanax pictus against Gastric Injury. Biomolecules & Therapeutics 23(1): 53–9.
Li, D.H., Y. Wang, Y.S. Lv, J.H. Liu, L. Yang, S.K. Zhang, and Y.Z. Zhuo. 2015. Preparative Purification of Liriodendrin from Sargentodoxa cuneata by Macroporous Resin. Biomedical Research International 2015: 861256.
Rittirsch, D., M.S. Huber-Lang, M.A. Flierl, and P.A. Ward. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36.
Zhong, W.T., Y.C. Wu, X.X. Xie, X. Zhou, M.M. Wei, L.W. Soromou, et al. 2013. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia 90: 132–139.
Dombrovskiy, V.Y., A.A. Martin, J. Sunderram, and H.L. Paz. 2007. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical Care Medicine 35: 1244–1250.
Rubenfeld, G.D. 2003. Epidemiology of acute lung injury. Critical Care Medicine 31: 276–84.
Deitch, E.A. 2005. Rodent models of intra-abdominal infection. Shock 24(Suppl 1): 19–23.
Remick, D.G., D.E. Newcomb, G.L. Bolgos, and D.R. Call. 2000. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13: 110–116.
Rittirsch, D., L.M. Hoesel, and P.A. Ward. 2007. The disconnect between animal models of sepsis and human sepsis. Journal of Leukocyte Biology 81: 137–143.
Hirano, Y., M. Aziz, W.L. Yang, Z. Wang, M. Zhou, M. Ochani, A. Khader, and P. Wang. 2015. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Critical Care 19: 53.
Zhang, X., H. Huang, T. Yang, et al. 2010. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 41: 746.
Pages, G., and J. Pouyssegur. 2005. Transcriptional regulation of the Vascular Endothelial Growth Factor gene—a concert of activating factors. Cardiovascular Research 65: 564–73.
Leiser, S.F., and M. Kaeberiein. 2010. A role of sirt1 in the hypoxic response. Molecular Cell 38: 779–80.
Lim, J.H., Y.M. Lee, Y.S. Chun, J. Chen, J.E. Kim, and J.W. Park. 2010. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Molecular Cell 38: 864–78.
Kim, D.H., K.T. Lee, E.A. Bae, M.J. Han, and H.J. Park. 1999. Metabolism of liriodendrin and syringin by human intestinal bacteria and their relation to in vitro cytotoxicity. Archives of Pharmacal Research 22(1): 30–4.
Cho, S., M. Cho, J. Kim, M. Kaeberlein, S.J. Lee, and Y. Suh. 2015. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget 6(1): 43–55.
Park, H.W., S.Y. Cho, H.H. Kim, B.S. Yun, J.U. Kim, S.J. Lee, and J. Park. 2015. Enantioselective induction of SIRT1 gene by syringaresinol from Panax ginseng berry and Acanthopanax senticosus Harms stem. Bioorganic and Medicinal Chemistry Letters 25(2): 307–9.
Yamazaki, T., S. Shimosaka, H. Sasaki, T. Matsumura, T. Tukiyama, and T. Tokiwa. 2007. (+)-Syringaresinol-di-O-beta-D glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-kappaB activities. Toxicology In Vitro 21(8): 1530–7.
Barichello, T., J.J. Fortunato, A.M. Vitali, G. Feier, A. Reinke, J.C. Moreira, J. Quevedo, and F. Dal-Pizzol. 2006. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Critical Care Medicine 34(3): 886–889.
Chuang, C.C., S.C. Shiesh, C.H. Chi, Y.F. Tu, L.I. Hor, C.C. Shieh, and M.F. Chen. 2006. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Critical Care 10(1): R36.
Chtourou, Y., B. Aouey, M. Kebieche, and H. Fetoui. 2015. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-kB and P53 signaling pathways. Chemico-Biological Interactions 239: 76–86.
Vachharajani, V.T., T. Liu, X. Wang, J.J. Hoth, B.K. Yoza, and C.E. McCall. 2016. Sirtuins Link Inflammation and Metabolism. Journal of Immunology Research 2016: 8167273.
Kauppinen, A., T. Suuronen, J. Ojala, K. Kaarniranta, and A. Salminen. 2013. Antagonistic crosstalk between NF-kB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular Signalling 25(10): 1939–48.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No. 81470263) for their great support in financing these researches.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, L., Li, D., Zhuo, Y. et al. Protective Role of Liriodendrin in Sepsis-Induced Acute Lung Injury. Inflammation 39, 1805–1813 (2016). https://doi.org/10.1007/s10753-016-0416-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0416-1