Abstract
Cardiopulmonary bypass (CPB) is related to inflammatory response and pulmonary dysfunction. The aim of this study was to evaluate the effects of CPB leukocyte filtration on inflammation and lung function after coronary artery bypass grafting (CABG). A prospective randomized study was performed to compare CABG patients undergoing CPB leukocyte filtration (n = 9) or standard CPB (n = 11). Computed tomography, oxygenation, leukocyte count, hemodynamic data, PaO2/FiO2, shunt fraction, interleukins, elastase, and myeloperoxidase were evaluated. Data were analyzed using two-factor ANOVA for repeated measurements. The filtered group showed lower neutrophil counts up to 50 min of CPB, lower shunt fraction up to 6 h after surgery, and lower levels of IL-10 at the end of surgery (p < 0.05). There was no statistically significant difference between groups related to other parameters. Leukodepletion during CPB results in neutrophil sequestration by a short time, decreased IL-10 serum levels, and lower worsening of lung function only temporarily.
Similar content being viewed by others
INTRODUCTION
Changes in pulmonary function observed after coronary surgery with cardiopulmonary bypass (CPB) are related to increases in postoperative morbidity and mortality, principally if prolonged mechanical ventilation is required [1]. The systemic and pulmonary inflammatory response plays an important role in this multifactorial process [2–4]. Leukocyte activation and consequent alterations in endothelial integrity lead to changes in vascular permeability and resistance, which can worsen the ventilation/perfusion ratio and interfere with postoperative outcome [2, 3, 5]. Different kinds of pharmacological therapy, CPB circuits, and lung-protective mechanical ventilation strategies have been used in order to decrease the inflammatory response, although the mechanism of each is incompletely understood [2, 4, 6]. Additionally, it is necessary to consider the secondary effects on pulmonary and systemic microcirculation.
Leukocyte depletion during CPB, although controversial [7], can reduce unwanted inflammatory response [8, 9], decreasing IL-6 and IL-8 activity, with possible improvement in hemodynamic parameters and the oxygenation index [9–11]. On the other hand, the effect of filtration on anti-inflammatory levels of interleukins such as IL-10 and IL-1rA is not very clear. As IL-10 anti-inflammatory activity is partially triggered by downregulation of leukocyte expression of the major histocompatibility class II molecule (HLA-DR) [12], the level of this mediator can reflect the balance of pro- and anti-inflammatory cytokine expression, an aspect which deserves better understanding [13]. In inflammatory conditions, increased IL-10 levels can lead to a worsened clinical outcome [14, 15], although individual characteristics of IL-10 secretion, as a consequence of genetic polymorphism in its promoter, have been implicated in variable patterns of its expression [14]. We, therefore, hypothesized that leukocyte filtration during CPB could decrease the magnitude of systemic inflammatory response in individuals undergoing coronary artery bypass grafting, contributing to better postoperative morbidity.
The aims of this study were to evaluate if the leukocyte filter could reduce the inflammatory response proportionally to reduction in WBC count and what could be the impact on postoperative lung function, accessed by mediators of pro- and anti-inflammatory activity, extravascular lung water, loss of aeration, and oxygenation.
METHODS
After the approval statement of the ethics committee for this study (Ethical Committee No. 216/04), provided by the ethics committee for review of research projects (CAPPesq) of Clinics Hospital of the Medical School of the University of São Paulo, São Paulo, Brazil, a prospective randomized study was performed for evaluation of individuals undergoing CABG who had their physical state classified as PII or PIII, according to the American Society of Anesthesiologists (ASA) [16]. Surgical risk was stratified according to the Parsonnet criteria [17], and only those individuals considered to be of low to moderate risk were admitted. Subjects older than 70 years or those presenting with body mass index (BMI) over 35 kg/m2, with congestive heart failure (CHF) greater than class III (NYHA) [18], or with left ventricle ejection fraction less than 40 %; who had recently submitted to other surgery; who had creatinine levels ≥1.4 mg/dL; or who were using oral anticoagulants were all excluded.
Anesthesia Procedures
After signing the free and clarified consent form, subjects were evaluated with respect to demographic data, personal history, and blood samples. Patients received midazolam at a dose of 0.1 to 0.2 mg/kg orally, 30 min before surgery, to a maximum dose of 15 mg. After admission to the operating room, they were monitored through pulse oximetry, five-lead electrocardiogram, and continuous ST segment analysis (Siemens, Berlin, Germany). Peripheral venipuncture was obtained in the upper limb with a 14G or 16G catheter. Invasive blood pressure monitoring was performed after radial artery puncture with a 20G catheter, using a pressure transducer, and verifying attainment of the pressure curve (Siemens monitor SC 9000 Infinity XL, Munich, Germany). After preoxygenation with 100 % oxygen, intravenous infusion with 0.5 μg/kg sufentanil and 0.2 mg/kg etomidate was performed, and muscle relaxation was obtained with 0.1–0.2 mg/kg pancuronium bromide, followed by manual mask ventilation and tracheal intubation with a canula of suitable diameter. Mechanical ventilation was performed in a circular valve system with a carbon dioxide absorber, according to NBR/ABNT No. 10012 (Brazilian Association of Technical Standards), by a microprocessed electronic fan from a set of anesthesia equipment (Cicero®, Dräger and Siemens Company, Lübeck, Germany). It was adjusted to a volume chain (VC) of 8 mL/kg, FiO2 of 0.6, I/E ratio of 1:2, respiratory rate (RR) of 12 breaths/min (bpm), and positive end-expiratory pressure (PEEP) of 5 cm H2O and adjusted to maintain PETCO2 between 35 and 40 mmHg. Anesthesia was maintained with isoflurane inhalation adjusted between 0.5 and 1.0 MAC, and continuous infusion of sufentanil at 0.5 μg/kg h. During CPB, ventilation was interrupted and hypnosis was maintained by propofol infusion at a target-controlled concentration of 2.5 μg/mL.
After general anesthesia induction, nasopharynx temperature sensor placement, bladder catheterization for diuresis control, and pulmonary artery catheterization (7.5F catheter with thermal filament—CCO catheter, Baxter Edwards Critical Care, Irvine, CA, USA) connected to a Vigilance monitor (Baxter Edwards Critical Care, Irvine, CA, USA) were carried out.
Randomization and CPB Management
For the CPB pump, a non-heparinized roller circuit (Braille, São Jose do Rio Preto, Brazil) was used, filled with Ringer's solution to a total volume of 1,500 mL, and maintained near an average flux of 3,500 to 4,500 (3.5–4.5 L/m2 min), according to immediate need. Mild hypothermia between 28 and 32 °C was maintained; a membrane-type oxygenator was also used. Heparin (500 U/kg) was applied as an anticoagulant for CPB installation, and protamine was used to reverse the heparin's effect. The antifibrinolytic agent used was epsilon-aminocaproic acid (80 mg/kg); cardioplegia consisted of standard cardioplegic solution, added to the mixture of blood drained from the patient with priming contained in the reservoir.
Individuals were allocated randomly into the “control” or “filtered” groups according to simple randomization occurring immediately before surgery. In the filtered group, an additional leukocyte filter (Pall Biomedical Product, East Hills, NY, USA) was placed alongside the standard filter, which was clamped during CPB. In the control group, only the standard filter was used.
At the time of rewarming under CPB, inotropes or vasodilators were introduced by the anesthesiologist. At the end of the surgery, patients were transferred to the surgical intensive care unit.
Data Collection
A fast spiral thoracic CT scan (Aquilion 64, Toshiba Medical Systems, Otawara, Japan) was obtained during a 10-s period of apnea at the end of a normal expiration, with 10-mm cuts without interval, preoperatively and on the first postoperative day, to measure lung density along the sectional area, with evaluation of well, poorly, or non-aerated regions of the lung.
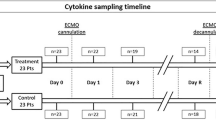
Hemodynamic data and the PaO2/FiO2 index value were obtained preoperatively (Pre-op.), after induction of anesthesia (After induction), 5 min before CPB (Beginning of CPB), 5 min after End of CPB (End of CPB), during skin suturing (End of surgery), and at 6 (6 h P.O.), 12 (12 h P.O.), and 24 h (24 h P.O.) after surgery. Intrapulmonary shunt fraction (Qs/Qt), TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-1rA, elastase, and myeloperoxidase (MPO) were also evaluated at the following times: After induction, Beginning of CPB, End of CPB, End of surgery, and at 6, 12, and 24 h P.O. Cell counts were performed at the following times: after induction, after 5 min of CPB (5 min CPB), after 25 min of CPB (25 min CPB), after 50 min of CPB (50 min CPB), during suturing (End surgery), and at 12 and 24 h P.O. The presence and type of any infection were recorded postoperatively.
Sample Size Calculation and Statistical Analysis
The sample size was calculated based on the assumption that, when CPB started, the first white blood cell count would have a numerical reduction of at least 30 % pre-CPB, which, in turn, was considered taking as reference an average of 5,000 cells, with a standard deviation of 900 [19, 20]. During CPB, still in relation to the first cell count, a confidence interval of 95 % was considered, with an interval length of 600, which generated an n of 8 individuals in the filtered group.
Quantitative and demographic variables were descriptively presented in tables with means and standard deviations. Where indicated, t test was carried out for independent samples.
Analysis of variance (ANOVA) for repeated measurements was used to verify differences between groups. The method of ANOVA included the factor group, time, and interaction between time and group. For those tests which showed statistically significant differences (p < 0.05), we followed up with the Student–Newman–Keuls test for multiple comparisons between groups and times.
RESULTS
A total of 26 individuals were initially assessed for eligibility of participation, but only 20 completed the study: 11 in the control group and 9 in the filtered group (Fig. 1). One did not meet the inclusion criteria during the interview. Another refused to participate after the interview. Twenty-four individuals were randomized: 12 to the control group and 12 to the filtered group. Two individuals in the filtered group did not receive the allocated intervention. Another was excluded from the final analysis, which resulted at the end in nine individuals. In the control group, 1 individual was excluded from the final analysis, resulting in 11 individuals. The groups were comparable concerning demographic data, length of CPB, and hemodynamic data (Tables 1 and 2). Although the total leukocyte count did not present explicit differences between groups during CPB (p = 0.084), we observed a greater decrease in the neutrophil count in the filtered group than in the control group, from the beginning to 50 min of CPB (p = 0.036), as shown in Figs. 2 and 3.
Regarding respiratory data, both groups presented slight reductions in PaO2/FiO2, from the induction of anesthesia to the end of surgery, with a gradual restoration of normal values up to 24 h after the end of surgery (Fig. 4), without differences between the groups. However, from the end of CPB to 6 h after surgery, the rise in Qs/Qt was less in the filtered group than in the control group (p = 0.040), with a tendency to return to baseline in both groups on the first postoperative day (Fig. 5).
As shown in Table 3, TNF-α and IL-1β serum levels did not present significant changes over time or between groups. For IL-6, IL-8, and IL-1rA, an increase over time was observed without differences between groups, the same for enzymes regulating cell degranulation, elastase, and MPO. On the other hand, IL-10 presented a significant difference between the control and filtered groups (p = 0.031). IL-10 serum levels were notably lower in the filtered group than in the control group from the end of CPB (p < 0.001) to the end of surgery (p = 0.002); thereafter, there was a reduction in these values with time in both groups (Fig. 6).
Regarding parameters derived from the chest helical CT on the first postoperative day, compared to preoperative values (Table 4), the filtered and control groups both presented increases in lung weight (p = 0.001 and p = 0.004, respectively). However, the results derived from entire lung volume (gas plus tissue) showed reductions over time in the control group (p = 0.002), but not in the filtered group (p = 0.083). These data reflect a decline in gas volume after surgery, but no differences between the groups were observed in either lung weight or lung volume (p = 0.379 and p = 0.220, respectively). In addition, the incremental changes in lung weight, represented as gains in lung tissue, were observed with no statistically significant difference between groups (p = 0,250), which can be attributed to increased extravascular lung water observed postoperatively.
DISCUSSION
The results of this study showed that leukocyte filtration during CPB promoted a decrease in the neutrophil count, proportionally more evident than any change in the total leukocyte count, suggesting the effectiveness of the filter on activated polymorphonuclear cells. Reduced IL-10 expression in the filtered group was also observed. However, the beneficial effects on respiratory function were transient, with a reduced increase in the shunt fraction, suggesting a limited filtration capacity over the time it was used.
Regarding evaluation of the filter's effectiveness, the technique of its use during CPB can lead to different results in both clinical and experimental studies [7, 20–25]. Differences in filterability can be found when using one versus more than one filter, when the filter is used in the arterial versus the venous line or when it is used only part of the time versus for the total duration of CPB [8, 10, 26, 27]. With arterial line filtration, successful neutrophil reduction was time dependent, with limited improvement in lung function [8, 22]. On the other hand, a decrease in CPB flow can improve the efficiency of the filter in neutrophil depletion, resulting in diminished extravascular lung water and an absence of focal cellular injury signs as a result of reduced pulmonary sequestration of leukocytes, implying an improved arterial partial pressure of oxygen [26]. In clinical trials [28], flow variations imposed by the weight of individuals and surgical conditions, as well non-standard duration times for CPB, seemed to negatively affect an appropriate assessment of filter efficacy, compared with experimental models [26]. Such findings are probably responsible for the differences found between the current study and other reports [29]. We believe that definitive guidelines for when to use a leukocyte filter in the CPB circuit still remain to be determined, although some studies have discouraged its routine use [29, 30].
While the reduced PaO2/FiO2 ratio was unrelated to leukocyte filtration, the shunt fraction exhibited a less notable increase, not exceeding the early postoperative period, indicating better preservation of lung function. This observed effect on oxygenation could be related to a decrease in surfactant synthesis, resulting in lung collapse, especially in the dorsal part [31]. Depletion of activated cells can prevent endothelial injury, with reduced alveolar infiltration and a slight change in the vascular response mechanisms and pulmonary shunt [32]. However, the transience of the filtration effect is reinforced by the absence of more dramatic changes in the atelectasis grade found on the first postoperative day.
In that regard, post-CPB lung injury was characterized at CT scan as having a reduction in cephalocaudal dimensions, secondary to a decreased gas volume and increased tissue volume [33]. A postoperative volumetric loss pattern was observed in the control group but not in the filtered group. However, the drop of 22 % in total aeration in the control group was similar to the 17 % reduction in the filtered group. As the postoperative CT was performed on the first day after surgery, the result observed also reveals the transient effect of the leukocyte filtration. The clinical limitation of performing CT immediately after surgery, when the shunt fraction was greater in the control group, prevented the identification of differences in gas aeration patterns between the groups.
A similar evaluation of hemodynamic data was observed between groups, with increases in heart rate and cardiac output and decreases in systemic vascular resistance, without a significant change in PVR over the time. The artificial control of hemodynamic data by volume replacement and vasoactive drug infusion after CPB could prevent expression of the effect of leukocyte filtration in the cardiovascular system, as observed in other clinical studies of leukodepletion [27].
The reduced expression of IL-10 observed in the filtered group may represent a protective effect of this technique on the lungs, contributing to the transient effect of leukocyte filtration on the intrapulmonary shunt fraction. The anti-inflammatory activity of IL-10 is related to a decreased recognition of the surface antigen, with consequences for cellular adhesion, predisposing to an immunosuppressive state [12]. Levels of IL-10 are related to the severity of surgical trauma [13] and the sepsis state [34] and interfere with morbidity after coronary syndromes and myocarditis [35]. Corticosteroids can diminish pro-inflammatory cytokine release and increase blood IL-10 levels after CPB [36]. Nevertheless, in the current study, methylprednisolone was administered to both groups and so could not explain the observed difference between the groups. Thus, our results suggest that the filtered group was protected from the inflammatory insult of CPB, which could represent an advantage of this method, suggesting a smaller organic insult and reduced anti-inflammatory response. Furthermore, increased levels of IL-1rA can follow IL-10 elevation [12], as observed in the current study in the control group, suggesting a greater intensity of the anti-inflammatory response. On the other hand, the transient effect of filtration had no effect on the behavior of levels of the other interleukins. So, to provide more complete information on this topic, further investigations, with a sample size calculation based on the IL-10, could better discuss the relationship of this cytokine with leukodepletion, something not done by us.
Although we found higher elastase and myeloperoxidase levels after leukodepletion, this has previously been observed [7] and, as in our findings, did not result in a worsening of the oxygenation parameters. Conversely, decreased plasmatic elastase and pulmonary myeloperoxidase values were observed when filtration was done for a short period of time during CPB, thus resulting in a better oxygenation index (PaO2/FiO2) 24 h after surgery [9] and indicating that the filtration duration and the CPB apparatus were responsible for these results. In that sense, we believe that our duration time of filtration of 50 min during CPB may be responsible for the absence of more dramatic changes in values after CPB in the filtered group, which could lead to statistical significance.
Besides filter characteristics, additional limitations of this study are related to sample size, which was calculated for evaluation of neutrophil count. Although neutrophil counts were decreased, the time dependence of the filter's efficiency, and variations in patients and CPB conditions, resulted in only a transient impact on pulmonary function and release of inflammatory mediators. Additional studies of filtered and non-filtered cells, and improvements in the technological characteristics of the leukocyte filter, could result in innovation and better clinical results. Considering what was our primary objective, the benefit observed could represent one possible strategy to be used along with other methods, such as decreasing intraoperative fluid overload and reducing blood transfusion and operative length, in order to decrease overall morbidity associated to surgical procedures using CPB.
In conclusion, leukocyte filtration during cardiopulmonary bypass, when using an arterial line filter with a flow of up to 5 L/min m2 during the entire duration of CPB, results in an effective neutrophil sequestration up to 50 min of the machine operation and in decreased IL-10 serum levels up to the end of surgery, protecting the lungs only temporarily against acute injury related to CPB.
References
Kollef, M.H., T. Wragge, and C. Pasque. 1995. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest 107: 1395–1401.
Ranieri, V.M., P.M. Suter, C. Tortorella, et al. 1999. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama 282: 54–61.
Cremer, J., M. Martin, H. Redl, et al. 1996. Systemic inflammatory response syndrome after cardiac operations. Annals of Thoracic Surgery 61: 1714–1720.
Laffey, J.G., J.F. Boylan, and D.C. Cheng. 2002. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 97: 215–252.
Donnelly, S.C., R.M. Strieter, S.L. Kunkel, et al. 1993. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341: 643–647.
Apostolakis, E.E., E.N. Koletsis, N.G. Baikoussis, et al. 2010. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. Journal of Cardiothoracic Surgery 5: 1.
Mihaljevic, T., M. Tonz, L.K. von Segesser, et al. 1995. The influence of leukocyte filtration during cardiopulmonary bypass on postoperative lung function. A clinical study. Journal of Thoracic and Cardiovascular Surgery 109: 1138–1145.
Hachida, M., N. Hanayama, T. Okamura, et al. 1995. The role of leukocyte depletion in reducing injury to myocardium and lung during cardiopulmonary bypass. Asaio Journal 41: M291–M294.
Tao, K., Q. An, K. Lin, et al. 2009. Which is better to preserve pulmonary function: short-term or prolonged leukocyte depletion during cardiopulmonary bypass? Journal of Thoracic and Cardiovascular Surgery 138: 1385–1391.
Asimakopoulos, G. 2002. The inflammatory response to CPB: the role of leukocyte filtration. Perfusion 17(Suppl): 7–10.
Sheppard, S.V., R.V. Gibbs, and D.C. Smith. 2004. Does the use of leucocyte depletion during cardiopulmonary bypass affect exhaled nitric oxide production? Perfusion 19: 7–10.
McBride, W.T., and S.J. McBride. 1998. The balance of pro- and anti-inflammatory cytokines in cardiac surgery. Current Opinion in Anaesthesiology 11: 15–22.
Giannoudis, P.V., F. Hildebrand, and H.C. Pape. 2004. Inflammatory serum markers in patients with multiple trauma. Can they predict outcome? Journal of Bone & Joint Surgery, British 86: 313–323.
Galley, H.F., P.R. Lowe, R.L. Carmichael, et al. 2003. Genotype and interleukin-10 responses after cardiopulmonary bypass. British Journal of Anaesthesia 91: 424–426.
Neidhardt, R., M. Keel, U. Steckholzer, et al. 1997. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. Journal of Trauma 42: 863–870. discussion 870–1.
ASA. 1963. New classification of physical status. Anesthesiology 24: 111.
Bernstein, A.D., and V. Parsonnet. 2000. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Annals of Thoracic Surgery 69: 823–828.
The Criteria Committee of the New York Heart Association. 1994. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston (Mass): Little, Brown & Co; p. 253–256.
Faymonville, M.E., J. Pincemail, J. Duchateau, et al. 1991. Myeloperoxidase and elastase as markers of leukocyte activation during cardiopulmonary bypass in humans. Journal of Thoracic and Cardiovascular Surgery 102: 309–317.
Gu, Y.J., A.J. de Vries, P.W. Boonstra, et al. 1996. Leukocyte depletion results in improved lung function and reduced inflammatory response after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 112: 494–500.
Alexiou, C., S. Sheppard, A. Tang, et al. 2006. Leukocytes-depleting filters preferentially remove activated leukocytes and reduce the expression of surface adhesion molecules during the simulated extracorporeal circulation of human blood. Asaio Journal 52: 438–444.
Alexiou, C., A.A. Tang, S.V. Sheppard, et al. 2004. The effect of leucodepletion on leucocyte activation, pulmonary inflammation and respiratory index in surgery for coronary revascularisation: a prospective randomised study. European Journal of Cardio-Thoracic Surgery 26: 294–300.
Lust, R.M., A.P. Bode, L. Yang, et al. 1996. In-line leukocyte filtration during bypass. Clinical results from a randomized prospective trial. Asaio Journal 42: M819–M822.
Ortolano, G.A., G.S. Aldea, K. Lilly, et al. 2002. A review of leukofiltration in cardiac surgery: the time course of reperfusion injury may facilitate study design of anti-inflammatory effects. Perfusion 17(Suppl): 53–62.
Patel, A.N., S.W. Sutton, S. Livingston, A. Patel, et al. 2003. Clinical benefits of leukocyte filtration during valve surgery. American Journal of Surgery 186: 636–639. discussion 639–40.
Bando, K., R. Pillai, D.E. Cameron, et al. 1990. Leukocyte depletion ameliorates free radical-mediated lung injury after cardiopulmonary bypass. Journal of Thoracic and Cardiovascular Surgery 99: 873–877.
Karaiskos, T.E., G.M. Palatianos, C.D. Triantafillou, et al. 2004. Clinical effectiveness of leukocyte filtration during cardiopulmonary bypass in patients with chronic obstructive pulmonary disease. Annals of Thoracic Surgery 78: 1339–1344.
Matheis, G., M. Scholz, A. Simon, et al. 2001. Timing of leukocyte filtration during cardiopulmonary bypass. Perfusion 16(Suppl): 31–37.
Warren, O., C. Alexiou, R. Massey, et al. 2007. The effects of various leukocyte filtration strategies in cardiac surgery. European Journal of Cardio-Thoracic Surgery 31: 665–676.
Whitaker, D.C., J.A. Stygall, S.P. Newman, et al. 2001. The use of leucocyte-depleting and conventional arterial line filters in cardiac surgery: a systematic review of clinical studies. Perfusion 16: 433–446.
Tenling, A., T. Hachenberg, H. Tyden, et al. 1998. Atelectasis and gas exchange after cardiac surgery. Anesthesiology 89: 371–378.
Olivencia-Yurvati, A.H., C.A. Ferrara, N. Tierney, N. Wallace, and R.T. Mallet. 2003. Strategic leukocyte depletion reduces pulmonary microvascular pressure and improves pulmonary status post-cardiopulmonary bypass. Perfusion 18(Suppl 1): 23–31.
Rodrigues, R.R., A.Y. Sawada, J.J. Rouby, et al. 2011. Computed tomography assessment of lung structure in patients undergoing cardiac surgery with cardiopulmonary bypass. Brazilian Journal of Medical and Biological Research 44: 598–605.
Theobaldo, M.C., H.V. Barbeiro, D.F. Barbeiro, et al. 2012. Hypertonic saline solution reduces the inflammatory response in endotoxemic rats. Clinics 67: 1463–1468.
Nishii, M., T. Inomata, H. Takehana, et al. 2004. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. Journal of the American College of Cardiology 44: 1292–1297.
Giomarelli, P., S. Scolletta, E. Borrelli, et al. 2003. Biagioli B. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Annals of Thoracic Surgery 76: 117–123.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: Clinicaltrials.gov identifier: NCT01469676.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/2.0/.
About this article
Cite this article
de Amorim, C.G., Malbouisson, L.M.S., da Silva, F.C. et al. Leukocyte Depletion During CPB: Effects on Inflammation and Lung Function. Inflammation 37, 196–204 (2014). https://doi.org/10.1007/s10753-013-9730-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9730-z