Abstarct
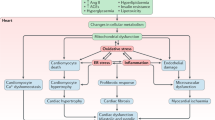
Diabetic complications are among the largely exigent health problems currently. Cardiovascular complications, including diabetic cardiomyopathy (DCM), account for more than 80% of diabetic deaths. Investigators are exploring new therapeutic targets to slow or abate diabetes because of the growing occurrence and augmented risk of deaths due to its complications. Research on rodent models of type 1 and type 2 diabetes mellitus, and the use of genetic engineering techniques in mice and rats have significantly sophisticated for our understanding of the molecular mechanisms in human DCM. DCM is featured by pathophysiological mechanisms that are hyperglycemia, insulin resistance, oxidative stress, left ventricular hypertrophy, damaged left ventricular systolic and diastolic functions, myocardial fibrosis, endothelial dysfunction, myocyte cell death, autophagy, and endoplasmic reticulum stress. A number of molecular and cellular pathways, such as cardiac ubiquitin proteasome system, FoxO transcription factors, hexosamine biosynthetic pathway, polyol pathway, protein kinase C signaling, NF-κB signaling, peroxisome proliferator-activated receptor signaling, Nrf2 pathway, mitogen-activated protein kinase pathway, and micro RNAs, play a major role in DCM. Currently, there are a few drugs for the management of DCM and some of them have considerable adverse effects. So, researchers are focusing on the natural products to ameliorate it. Hence, in this review, we discuss the pathogical, molecular, and cellular mechanisms of DCM; the current diagnostic methods and treatments; adverse effects of conventional treatment; and beneficial effects of natural product-based therapeutics, which may pave the way to new treatment strategies.

Graphical Abstract

Similar content being viewed by others
References
Aneja A, Tang W, Bansilal S et al (2008) Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121:748–757. https://doi.org/10.1016/j.amjmed.2008.03.046
Rubler S, Dlugash J, Yuceoglu YZ (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602. https://doi.org/10.1016/0002-9149(72)90595-4
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57:660–671. https://doi.org/10.1007/s00125-014-3171-6
Kohli S, Chhabra A, Jaiswal A, Rustagi Y, Sharma M, Rani V (2013) Curcumin suppresses gelatinase B mediated norepinephrine induced stress in H9C2 cardiomyocytes. PLoS One 8:76519. https://doi.org/10.1371/journal.pone.0076519
Alonso N, Moliner P, Mauricio D (2018) Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol 1067:197–217. https://doi.org/10.1007/5584_2017_105
WHO (2016) Global report on diabetes, ISBN 978 92 4 156525 7
Goyal BR, Mehta AA (2012) Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfunction. Hum Exp Toxicol 32:1–20. https://doi.org/10.1177/0960327112450885
Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang T, Huang X, Xiao J, Li Y, Li Z (2011) Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol 10:69. https://doi.org/10.1186/1475-2840-10-69
Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S (2013) Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol 165:494–498. https://doi.org/10.1016/j.ijcard.2011.09.005
Bodiga VL, Eda SR, Bodiga S (2014) Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev 19:49–63. https://doi.org/10.1007/s10741-013-9374-y
Sathibabu Uddandrao VV, Brahmanaidu P, Nivedha PR, Vadivukkarasi S, Saravanan G (2018) Beneficial role of some natural products to attenuate the diabetic cardiomyopathy through Nrf2 pathway in cell culture and animal models. Cardiovasc Toxicol 18:199–205. https://doi.org/10.1007/s12012-017-9430-2
Khan JN, Wilmot EG, Leggate M (2014) Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging 15:1263–1269. https://doi.org/10.1093/ehjci/jeu121
Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O (2014) Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol 2014:847827. https://doi.org/10.1155/2014/847827
Heiko Bugger DAE (2009) Rodent models of diabetic cardiomyopathy. Dis Model Mech 2:454–466. https://doi.org/10.1242/dmm.001941
Coort SL, Hasselbaink DM, Koonen DP et al (2004) Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 53:1655–1663. https://doi.org/10.2337/diabetes.53.7.1655
Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC (2005) Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288:2102–2110. https://doi.org/10.1152/ajpheart.00935.2004
Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M et al (2009) Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104:1085–1094. https://doi.org/10.1161/CIRCRESAHA.108.189316
Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED (2009) Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82:351–360. https://doi.org/10.1093/cvr/cvp017
Rennison JH, McElfresh TA et al (2009) Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol 46:883–890. https://doi.org/10.1016/j.yjmcc.2009.02.019
Ahmed SS, Jafer GA, Narang RM et al (1975) Preclinical abnormality of left ventricular function in diabetes mellitus. Am Heart J 89:153–158. https://doi.org/10.1016/0002-8703(75)90039-3
Fang ZY, Najos-Valencia O, Leano R, Marwick TH (2003) Patients with early diabetic heart disease demonstrate a normal myocardial response to dobutamine. J Am Coll Cardiol 42:46–53. https://doi.org/10.1016/S0735-1097(03)00654-5
Thompson GR, Partridge J (2004) Coronary calcification score: the coronary-risk impact factor. Lancet 363:557–559. https://doi.org/10.1016/S0140-6736(04)15544-X
Ficaro EP, Corbett JR (2004) Advances in quantitative perfusion SPECT imaging. J Nucl Cardiol 11:62–70. https://doi.org/10.1016/j.nuclcard.2003.10.007
Schinkel AF, Bax JJ, Valkema R, Elhendy A, van Domburg R, Vourvouri EC, Bountioukos MA, Krenning EP, Roelandt JR, Poldermans D (2003) Effect of diabetes mellitus on myocardial 18F-FDG SPECT using acipimox for the assessment of myocardial viability. J Nucl Med 44:877–883
Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C (2001) Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res 89:684–691. https://doi.org/10.1161/hh2001.097797
Coats AJ, Anker SD (2000) Insulin resistance in chronic heart failure. J Cardiovasc Pharmacol 35:9–14. https://doi.org/10.1016/j.jacc.2008.03.044
Stanley WC, Marzilli M (2003) Metabolic therapy in the treatment of ischaemic heart disease: the pharmacology of trimetazidine. Fundam Clin Pharmacol 17:133–145. https://doi.org/10.1046/j.1472-8206.2003.00154.x
Turan B (2010) Role of antioxidants in redox regulation of diabetic cardiovascular complications. Curr Pharm Biotechnol 11:819–836. https://doi.org/10.2174/138920110793262123
Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF (2006) Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther 111:508–531. https://doi.org/10.1016/j.pharmthera.2005.11.001
Brahmanaidu P, Sathibabu Uddandrao VV, Saravanan G (2017) Reversal of endothelial dysfunction in aorta of streptozotocinnicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Mol Cell Biochem 432:25–32. https://doi.org/10.1007/s11010-017-2994-0
Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J (2001) Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 50:2363–2375. https://doi.org/10.2337/diabetes.50.10.2363
Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG (2003) Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart 89:449–451. https://doi.org/10.1136/heart.89.4.449
Argirova MD, Ortwerth BJ (2003) Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys 420:176–184. https://doi.org/10.1016/j.abb.2003.09.005
Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, Perry CN, Dong XC, Valdez S, Rech M, Sheng X, Weimer BC, Gottlieb RA, White MF, Abel ED (2013) Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest 123:5319–5333. https://doi.org/10.1172/JCI71171
Lakshmanan AP, Harima M, Suzuki K, Soetikno V, Nagata M, Nakamura T, Takahashi T, Sone H, Kawachi H, Watanabe K (2013) The hyperglycemia stimulated myocardial endoplasmic reticulum (ER) stress contributes to diabetic cardiomyopathy in the transgenic nonobese type 2 diabetic rats: a differential role of unfolded protein response (UPR) signaling proteins. Int J Biochem Cell Biol 45:438–447. https://doi.org/10.1016/j.biocel.2012.09.017
Frieler RA, Mortensen RM (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131:1019–1030. https://doi.org/10.1161/CIRCULATIONAHA.114.008788
Li J, Ma W, Yue G, Tang Y, Kim IM, Weintraub NL, Wang X, Su H (2017) Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy. J Mol Cell Cardiol 102:53–60. https://doi.org/10.1016/j.yjmcc.2016.11.013
Asghar O, Al-Sunni A, Khavandi K, Withers S et al (2009) Diabetic cardiomyopathy. Clin Sci 116:741–760. https://doi.org/10.1042/CS20080500
Tang M, Huang Li J et al (2010) Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotesmaladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88:424–433. https://doi.org/10.1093/cvr/cvq217
Ferdous A, Battiprolu PK, Ni YG, Rothermel BA, Hill JA (2010) FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res 3:355–364. https://doi.org/10.1007/s12265-010-9200-z
Marsh SA, DellItalia LJ, Chatham JC (2011) Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40:819–828. https://doi.org/10.1007/s00726-010-0699-8
Wollaston-Hayden EE, Harris RB, Liu B, Bridger R, Xu Y et al (2015) Global O-GlcNAc levels modulate transcription of the adipocyte secretome during chronic insulin resistance. Front Endocrinol (Lausanne) 5:223. https://doi.org/10.3389/fendo.2014.00223
Rajamani U, Essop MF (2010) Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis. Am J Physiol Cell Physiol 299:139–147. https://doi.org/10.1152/ajpcell.00020.2010
Gabbay KH (1973) The sorbitol pathway and the complications of diabetes. N Engl J Med 288:831–836. https://doi.org/10.1056/NEJM197304192881609
Brahmanaidu P, Sathibabu Uddandrao VV, Pothani S, Saravanan G et al (2016) Effects of S-allylcysteine on biomarkers of polyol pathway in experimental type II diabetes in rats. Can J Dia 40:442–448. https://doi.org/10.1016/j.jcjd.2016.03.006
Huynh K, Bianca C et al (2014) Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 142:375–415. https://doi.org/10.1016/j.pharmthera.2014.01.003
Bernardo BC, Weeks KL, Pretorius L, McMullen JR (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128:191–227. https://doi.org/10.1016/j.pharmthera.2010.04.005
Palomer XL, Salvado E, Barroso Vazquez-Carrera M (2013) An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 168:3160–3172. https://doi.org/10.1016/j.ijcard.2013.07.150
Turner NA, Mughal RS, Warburton P et al (2007) Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc Res 76:81–90. https://doi.org/10.1016/j.cardiores.2007.06.003
Duncan JG (2011) Peroxisome proliferator activated receptor-alpha (PPAR alpha) and PPAR gamma coactivator-1alpha (PGC-1 alpha) regulation of cardiac metabolism in diabetes. Pediatr Cardiol 32:323–328. https://doi.org/10.1007/s00246-011-9889-8
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP (2007) Nuclear receptors PPAR beta/delta and PPAR alpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. https://doi.org/10.1172/JCI32578
Luo J, Wu S, Liu J, Li Y, Yang H, Kim T, Zhelyabovska O, Ding G, Zhou Y, Yang Y, Yang Q (2010) Conditional PPARgamma knockout from cardiomyocytes of adult mice impairs myocardial fatty acid utilization and cardiac function. Am J Transl Res 3:61–72
Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105:365–374. https://doi.org/10.1161/CIRCRESAHA.109.199919
Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L (2014) Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol 77:42–52. https://doi.org/10.1016/j.yjmcc.2014.09.022
Marber MS, Rose B, Wang Y (2011) The p38 mitogen-activated protein kinase pathway-a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol 51:485–490. https://doi.org/10.1016/j.yjmcc.2010.10.021
Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG (2009) The signal transduction pathway of PKC/NF-κB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol 8(1):8. https://doi.org/10.1186/1475-2840-8-8
Bernardo BC, Charchar FJ, Lin RC, McMullen JR (2012) A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ 21:131–142. https://doi.org/10.1016/j.hlc.2011.11.002
Kartha RV, Subramanian S (2010) MicroRNAs in cardiovascular diseases: biology and potential clinical applications. J Cardiovasc Transl Res 3:256–270. https://doi.org/10.1007/s12265-010-9172-z
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105. https://doi.org/10.1101/gr.082701.108
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P (2016) Micro- RNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471:423–429. https://doi.org/10.1016/j.bbrc.2016.02.065
Raut SK, Kumar A, Singh GB, Nahar U, Sharma V, Mittal A, Sharma R, Khullar M (2015) miR-30c mediates upregulation of Cdc42 and Pak1 in diabetic cardiomyopathy. Cardiovasc Ther 33:89–97. https://doi.org/10.1111/1755-5922.12113
Li X, Du N, Zhang Q et al (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5:1479. https://doi.org/10.1038/cddis.2014.430
Von Bibra H, Siegmund T, Hansen A et al (2007) Augmentation of myocardial function by improved glycemic control in patients with type 2 diabetes mellitus. Dtsch Med Wochenschr 132:729–734. https://doi.org/10.1055/s-2007-973608
Konduracka E, Gackowski A, Rostoff P, Galicka-Latala D, Frasik W, Piwowarska W (2007) Diabetes-specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J 28:2465–2471. https://doi.org/10.1093/eurheartj/ehm361
Nguyen NDT, Le LT (2012) Targeted proteins for diabetes drug design. Adv Nat Sci Nanosci Nanotechnol 3:1–9. https://doi.org/10.1088/2043-6262/3/1/013001
Tiwari N, Thakur AK, Kumar V, Dey A, Kumar V (2014) Therapeutic targets for diabetes mellitus: an update. Pharmacol Biopharm 3:117. https://doi.org/10.4172/2167-065X.1000117
Lebovitz HE (1997) Alpha-Glucosidase inhibitors. Endocrinol Metab Clin N Am 26:539–551. https://doi.org/10.1016/S0889-8529(05)70266-8
Andreadis EA, Katsanou PM, Georgiopoulos DX, Tsourous G, Yfanti G, Gouveri E, Diamantopoulos E (2009) The effect of metformin on the incidence of type 2 diabetes mellitus and cardiovascular disease risk factors in overweight and obese subjects the Carmos study. Exp Clin Endocrinol Diabetes 117:175–180. https://doi.org/10.1055/s-0028-1087177
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778. https://doi.org/10.2337/db10-0351
Young LH (2003) Insulin resistance and the effects of thiazolidinediones on cardiac metabolism. Am J Med 115:75–80. https://doi.org/10.1016/j.amjmed.2003.09.013
Zaman AK, Fujii S, Goto D, Furumoto T, Mishima T, Nakai Y, Dong J, Imagawa S, Sobel BE, Kitabatake A (2004) Salutary effects of attenuation of angiotensin ii on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol 37:525–535. https://doi.org/10.1016/j.yjmcc.2004.05.006
Adeghate E, Kalasz H, Veress G, Teke K (2010) Medicinal chemistry of drugs used in diabetic cardiomyopathy. Curr Med Chem 17:517–551. https://doi.org/10.2174/092986710790416281
Kawasaki D, Kosugi K, Waki H, Yamamoto K, Tsujino T, Masuyama T (2007) Role of activated renin-angiotensin system in myocardial fibrosis and left ventricular diastolic dysfunction in diabetic patients–reversal by chronic angiotensin ii type 1a receptor blockade. Circ J 71:524–529. https://doi.org/10.1253/circj.71.524
Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C (2007) Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56:641–646. https://doi.org/10.2337/db06-1163
Solomon SD, Skali H, Anavekar NS, Bourgoun M, Barvik S, Ghali JK, Warnica JW, Khrakovskaya M, Arnold JMO, Schwartz Y, Velazquez EJ, Califf RM, McMurray JV, Pfeffer MA (2005) Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 111:3411–3419. https://doi.org/10.1161/CIRCULATIONAHA.104.508093
Fang ZY, Prins JB, Marwick TH (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567. https://doi.org/10.1210/er.2003-0012
Veeraveedu PT, Watanabe K, Ma M, Gurusamy N, Palaniyandi SS, Wen J, Prakash P, Wahed MII, Kamal FA, Mito S, Kunisaki M, Kodama M, Aizawa Y (2006) Comparative effects of pranidipine with amlodipine in rats with heart failure. Pharmacology 77:1–10. https://doi.org/10.1159/000091746
Rajanikant GK, Zemke D, Kassab M, Majid A (2007) The therapeutic potential of statins in neurological disorders. Curr Med Chem 14:103–112. https://doi.org/10.2174/092986707779313462
Nisbet JC, Sturtevant JM, Prins JB (2004) Metformin and serious adverse effects. Med J Aust 180:53–54
Lund A, Knop FK (2012) Worry vs. knowledge about treatment-associated hypoglycaemia and weight gain in type 2 diabetic patients on metformin and/or sulphonylurea. Curr Med Res Opin 28:731–736. https://doi.org/10.1185/03007995.2012.681639
Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM (2008) Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57:1723–1729. https://doi.org/10.2337/db07-1472
Nicolle LE, Capuano G, Ways K, Usiskin K (2012) Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 28:1167–1171. https://doi.org/10.1185/03007995.2012.689956
fa V d l, Lucassen PL, Akkermans RP et al (2005) Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 28:154–163. https://doi.org/10.2337/diacare.28.1.154
Cobble M (2012) Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr 4:8. https://doi.org/10.1186/1758-5996-4-8
Ferwana M, Firwana B, Hasan R, al-Mallah MH, Kim S, Montori VM, Murad MH (2013) Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med 30:1026–1032. https://doi.org/10.1111/dme.12144
Campbell RK, Cobble ME, Reid TS, Shomali ME (2010) Distinguishing among incretin-based therapies. Safety, tolerability, and nonglycemic effects of incretin-based therapies. J Fam Pract 59:20–27
Kokil GR, Veedu RN, Ramm GA, Prins JB, Parekh HS (2015) Type 2 diabetes mellitus: limitations of conventional therapies and intervention with nucleic acid-based therapeutics. Chem Rev 115:4719–4743. https://doi.org/10.1021/cr5002832
Saravanan G, Ponmurugan P (2012) Antidiabetic effect of S-allylcysteine: effect on thyroid hormone and circulatory antioxidant system in experimental diabetic rats. J Diab Comp 26:280–285. https://doi.org/10.1016/j.jdiacomp.2012.03.024
Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovasc Hematol Agents Med Chem 15:71–77. https://doi.org/10.2174/1871525714666160418114120
Saravanan G, Ponmurugan P, Senthil kumar GP, Rajarajan T (2009) Modulatory effect of S-allylcysteine on glucose metabolism in streptozotocin induced diabetic rats. J Funct Foods 1:336–340. https://doi.org/10.1016/j.jff.2009.09.001
Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, Rochette L (2009) Antioxidant properties of an endogenous thiol: alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol 54:391–398. https://doi.org/10.1097/FJC.0b013e3181be7554
Li C-j, Lv L, Li H, Yu D-m (2012) Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 11:73. https://doi.org/10.1186/1475-2840-11-73
Li H, Xia N, Forstermann U (2012) Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26:102–110. https://doi.org/10.1016/j.niox.2011.12.006
Dolinsky VW, Dyck JR (2011) Calorie restriction and resveratrol in cardiovascular health and disease. Biochem Biophys Acta 1812:1477–1489. https://doi.org/10.1016/j.bbadis.2011.06.010
Guo S, Yao Q, Ke Z, Chen H, Wu J, Liu C (2015) Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol 412:85–94. https://doi.org/10.1016/j.mce.2015.05.034
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50:151–161. https://doi.org/10.1007/s00394-011-0188-1
Wei Y, Jiliang W, Fei C et al (2012) Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 7(52013):e52013. https://doi.org/10.1371/journal.pone.0052013
Xu K, Chu F, Li G, Xu X, Wang P, Song J, Zhou S, Lei H (2014) Oleanolic acid synthetic oligoglycosides: a review on recent progress in biological activities. Pharmazie 69:483–495. https://doi.org/10.1691/ph.2014.3933
Li WF, Wang P, Li H, Li TY, Feng M, Chen SF (2017) Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp Ther Med 14:848–854. https://doi.org/10.3892/etm.2017.4527
Agyemang K, Han L, Liu E, Zhang Y, Wang T, Gao X (2013) Recent advances in Astragalus membranaceus anti-diabetic research: pharmacological effects of its phytochemical constituents. Evid Based Complement Alternat Med 2013:654643. https://doi.org/10.1155/2013/654643
Chen W, Xia P et al (2012) Improvement of myocardial glycolipid metabolic disorder in diabetic hamster with Astragalus polysaccharides treatment. Mol Biol Rep 39:7609–7615. https://doi.org/10.1007/s11033-012-1595-y
Sun S, Yang S, Dai M, Jia X, Wang Q, Zheng Z, Mao Y (2017) The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complement Altern Med 17:310. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Yu Y, Zheng G (2017) Troxerutin protects against diabetic cardiomyopathy through NF κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep 15:3473–3478. https://doi.org/10.3892/mmr.2017.6456
Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF (2007) Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol 42:884–895. https://doi.org/10.1016/j.yjmcc.2006.12.018
Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD (2011) Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60:3055–3066. https://doi.org/10.2337/db11-0807
Tsuda H, Ohshima Y, Nomoto H, Fujita KI, Matsuda E, Iigo M, Takasuka N, Moore MA (2004) Cancer prevention by natural compounds. Drug Metab Pharmacokinet 19:245–263. https://doi.org/10.2133/dmpk.19.245
Yeh CT, Ching LC, Yen GC (2009) Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nut Biochem 20:163–171. https://doi.org/10.1016/j.jnutbio.2008.01.005
Leung L, Kwong M, Hou S, Lee C, Chan JY (2003) Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278:48021–48029. https://doi.org/10.1074/jbc.M308439200
Niu L, He XH, Wang QW, Fu MY, Xu F, Xue Y, Wang ZZ, An XJ (2015) Polyphenols in regulation of redox signaling and inflammation during cardiovascular diseases. Cell Biochem Biophys 72:485–494. https://doi.org/10.1007/s12013-014-0492-5
Li M, Jiang Y, Jing W, Sun B, Miao C, Ren L (2013) Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can J Physiol Pharmacol 91:951–959. https://doi.org/10.1139/cjpp-2012-0432
Panchal SK, Poudyal H, Brown L (2012) Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr 142:1026–1032. https://doi.org/10.3945/jn.111.157263
Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan T, Zhang F, Guo Y, Chen Y, Wu D (2015) Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol Appl Pharmacol 287:168–177. https://doi.org/10.1016/j.taap.2015.06.002
Gao L, Yao R, Liu Y, Wang Z, Huang Z, du B, Zhang D, Wu L, Xiao L, Zhang Y (2017) Isorhamnetin protects against cardiac hypertrophy through blocking PI3K-AKT pathway. Mol Cell Biochem 429:167–177. https://doi.org/10.1007/s11010-017-2944-x
Nakayama A, Morita H, Nakao T, Yamaguchi T, Sumida T, Ikeda Y, Kumagai H, Motozawa Y, Takahashi T, Imaizumi A, Hashimoto T, Nagai R, Komuro I (2015) A food-derived flavonoid luteolin protects against angiotensin II-induced cardiac remodeling. PLoS One 10(0137106):e0137106. https://doi.org/10.1371/journal.pone.0137106
Naidu PB, Uddandrao S, Naik RR et al (2016) Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol 419:139–147. https://doi.org/10.1016/j.mce.2015.10.007
Kalaivani A, Uddandrao VVS, Parim B et al (2018) Reversal of high fat diet-induced obesity through modulating lipid metabolic enzymes and inflammatory markers expressions in rats. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2018.1452036
Kalaivani A, Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G, Nivedha PR, Tamilmani P, Swapna K, Vadivukkarasi S (2017) Anti obese potential of Cucurbita maxima seeds oil: effect on lipid profile and histoarchitecture in high fat diet induced obese rats. Nat Prod Res. https://doi.org/10.1080/14786419.2017.1389939
Meriga B, Parim B, Chunduri VR, Naik RR, Nemani H, Suresh P, Ganapathy S, Uddandrao S (2017) Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nut Met 14:76. https://doi.org/10.1186/s12986-017-0228-9
Sathibabu Uddandrao VV, Brahmanaidu P, Meriga B, Saravanan G (2016) The potential role of S-allylcysteine as antioxidant against various disorders in animal models. Oxid Antioxid Med Sci 5:79–86. https://doi.org/10.5455/oams.240716.rv.025
Ghanshyam P, Chhayakanta P, Shankar MU et al (2012) Investigation of possible hypoglycemic and hypolipidemic effect of methanolic extract of Sesbania grandiflora. Int Res J Pharm 3:275–280
Nandi MK, Garabadu D, Krishnamurthy S, Singh TD, Singh VP (2014) Methanolic fruit extract of Sesbania grandiflora exhibits anti-hyperglycemic activity in experimental type-2 diabetes mellitus model. Ann Biol Res 5:50–58
Sangeetha A, Sriram Prasath G, Subramanian S (2014) Antihyperglycemic and antioxidant potentials of Sesbania grandiflora leaves studies in STZ induced experimental diabetic rats. Int J Pharm Sci Res 5:2266–2275. https://doi.org/10.13040/IJPSR.0975-8232.5(6).2266-75
Islam T, Rahman A, Islam AU (2013) In vitro effect of aqueous extract of fresh leaves of Abroma augusta L on the diffusion of glucose. Bangladesh Pharm J 16:21–26. https://doi.org/10.3329/bpj.v16i1.14486
Khanra R, Dewanjee S, Dua T, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M (2015) Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 13:6. https://doi.org/10.1186/s12967-014-0364-1
Wang C, Aungi HH, Zhang B et al (2008) Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res 28:2545–2552
Sen S, Chen S, Wu Y, Feng B, Lui E, Chakrabarti S (2012) Preventive effects of north American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother Res 27:290–298. https://doi.org/10.1002/ptr.4719
Padiya R, Banerjee SK (2013) Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric 5:105–127. https://doi.org/10.2174/18761429113059990002
Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh S, Gurtu S (2012) Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxidative Med Cell Longev 2012:374037. https://doi.org/10.1155/2012/374037
Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Intake of garlic and its bioactive components. J Nut 131:955–962. https://doi.org/10.1093/jn/131.3.955S
Hiramatsu K, Tsuneyoshi T, Ogawa T, Morihara N (2016) Aged garlic extract enhances heme oxygenase-1 and glutamate–cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nut Res 36:143–149. https://doi.org/10.1016/j.nutres.2015.09.018
Sun Z, Chin YE, Zhang DD (2009) Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29:2658–2672. https://doi.org/10.1128/MCB.01639-08
Khatua TN, Adela R, Banerjee SK (2013) Garlic and cardioprotection: insights into the molecular mechanisms. Can J Physiol Pharma 91:448–458. https://doi.org/10.1139/cjpp-2012-0315
Acknowledgements
The authors are thankful to Innovation in Science Pursuit for Inspired Research (INSPIRE), Department of Science & Technology (DST). Govt. of India for providing the financial assistance and faculty fellowship (Grant No: DST/INSPIRE/04/2016/000893). The authors are also thankful to Indian Council of Medical Research, Government of India for providing laboratory and library facilities. The authors are also thankful to Dr. Mir Irfan and Dr. Syed S.Y.H. Qadri, Dr. Harishankar Nemani for helpful comments, and Mr. Srinivas for technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Parim, B., Sathibabu Uddandrao, V.V. & Saravanan, G. Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev 24, 279–299 (2019). https://doi.org/10.1007/s10741-018-9749-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9749-1