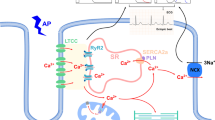
Abstract
Diabetic cardiomyopathy (DCM) is a diabetes mellitus–induced pathophysiological condition characterized by cardiac structural, functional, and metabolic changes that can result in heart failure (HF), in the absence of coronary artery disease, hypertension, and valvular heart disease. Metabolic alterations such as hyperglycemia, insulin resistance, hyperinsulinemia, and increased metabolism of free fatty acids result in oxidative stress, inflammation, advanced glycation end products formation, abnormalities in calcium homeostasis, and apoptosis that are responsible for structural remodeling. Cardiac stiffness, hypertrophy, and fibrosis eventually lead to dysfunction and HF with preserved ejection fraction and/or HF with reduced ejection fraction. In this review, we analyzed in detail the cellular and molecular mechanisms and the metabolic pathways involved in the pathophysiology of DCM. Different phenotypes are observed in DCM, and it is not clear yet if the restrictive and the dilated phenotypes are distinct or represent an evolution of the same disease. Phenotypic differences can be observed between T1DM and T2DM DCM, possibly explained by the different myocardial insulin action. Further studies are needed in order to better understand the underlying mechanisms of DCM and to identify appropriate therapeutic targets and novel strategies to prevent and reverse the progression toward heart failure in diabetic patients.



Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
International Diabetes Federation (2019) IDF Diabetes Atlas, 9th edition (Online). [Cited November 2, 2020]. Available from: https://www.diabetesatlas.org/en
Lind M, Svensson AM, Rosengren A (2015) Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 372(9):880–881. https://doi.org/10.1056/NEJMc1415677 (PMID: 25714168)
Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M (2015) Excess mortality among persons with type 2 diabetes. N Engl J Med 373:1720–1732 [PMID: 26510021 https://doi.org/10.1056/NEJMoa1504347]
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. The Framingham study. JAMA 241:2035–2038 [PMID: 430798 https://doi.org/10.1001/jama.241.19.2035]
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122:624–638 (PubMed: 29449364)
Rajbhandari J, Fernandez CJ, Agarwal M, Yeap BXY, Pappachan JM (2021) Diabetic heart disease: a clinical update. World J Diabetes 12(4):383–406
Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A (2015) Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J 36:94–99 [PMID: 25298237 https://doi.org/10.1093/eurheartj/ehu383]
Spallone V (2019) Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J 43:3–30 [PMID: 30793549 https://doi.org/10.4093/dmj.2018.0259]
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12:144–153. https://doi.org/10.1030/nrendo.2015.216
Seferović PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36(27):1718–27, 1727a-1727c. https://doi.org/10.1093/eurheartj/ehv134. Epub 2015 Apr 17. PMID: 25888006
Dillmann WH (2019) Diabetic Cardiomyopathy. Circ Res 124(8):1160–1162. https://doi.org/10.1161/CIRCRESAHA.118.314665.PMID:30973809;PMCID:PMC6578576
Evangelista I, Nuti R, Picchioni T, Dotta F, Palazzuoli A (2019) Molecular dysfunction and phenotypic derangement in diabetic cardiomyopathy. Int J Mol Sci 20(13):3264. https://doi.org/10.3390/ijms20133264.PMID:31269778;PMCID:PMC6651260
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114:597–605
Aronson D (2003) Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21(1):3–12
Aragno M, Mastrocola R, Medana C, Catalano MG, Vercellinatto I, Danni O, Boccuzzi G (2006) Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology 147(12):5967–5974. https://doi.org/10.1210/en.2006-0728 (Epub 2006 Aug 24 PMID: 16935841)
Li CJ, Lv L, Li H, Yu DM (2012) Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 19(11):73. https://doi.org/10.1186/1475-2840-11-73.PMID:22713251;PMCID:PMC3472273
Kaludercic N, Di Lisa F (2020) Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front Cardiovasc Med 18(7):12. https://doi.org/10.3389/fcvm.2020.00012.PMID:32133373;PMCID:PMC7040199
Bertero E, Maack C (2018) Calcium signaling and reactive oxygen species in mitochondria. Circ Res 122(10):1460–1478. https://doi.org/10.1161/CIRCRESAHA.118.310082 (PMID: 29748369)
Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, Zweier JL (2008) Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol 294:H1426–H1434. https://doi.org/10.1152/ajpheart.01016.2007
Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, King GL (2002) Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes 51:2709–2718
Lei S, Li H, Xu J et al (2013) Hyperglycemia-induced protein kinase C β2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 62(7):2318–2328. https://doi.org/10.2337/db12-1391
Palomer X, Pizarro-Delgado J, Vázquez-Carrera M (2018) Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci 39:452–467 [PMID: 29605388 https://doi.org/10.1016/j.tips.2018.02.010]
Granatiero V, De Stefani D, Rizzuto R (2017) Mithocondrial Calcium Handling in Physiology and Disease. Adv Exp Med Biol 982:25–47. https://doi.org/10.1007/978-3-319-55330-6_2
Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV (2017) Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced Thp1 monocytes and male mice. Endocrinology 158(10):3661–3675. https://doi.org/10.1210/en.2017-00294.PMID:28938395;PMCID:PMC5659696
Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL (2003) Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 285(6):R1481–R1489. https://doi.org/10.1152/ajpregu.00232.2003 (Epub 2003 Aug 28 PMID: 12947030)
von Lewinski D, Rainer PP, Gasser R, Huber MS, Khafaga M, Wilhelm B, Haas T, Mächler H, Rössl U, Pieske B (2010) Glucose-transporter-mediated positive inotropic effects in human myocardium of diabetic and nondiabetic patients. Metabolism 59(7):1020–1028. https://doi.org/10.1016/j.metabol.2009.10.025 (Epub 2009 Dec 31 PMID: 20045149)
Wang Y, Zhou S, Sun W, McClung K, Pan Y, Liang G, Tan Y, Zhao Y, Liu Q, Sun J, Cai L (2014) Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. Am J Physiol Endocrinol Metab 306(11):E1239–E1247. https://doi.org/10.1152/ajpendo.00629.2013 (Epub 2014 Apr 8 PMID: 24714399)
Barbati SA, Colussi C, Bacci L, Aiello A, Re A, Stigliano E, Isidori AM, Grassi C, Pontecorvi A, Farsetti A, Gaetano C, Nanni S (2017) Transcription factor CREM mediates high glucose response in cardiomyocytes and in a male mouse model of prolonged hyperglycemia. Endocrinology 158(7):2391–2405. https://doi.org/10.1210/en.2016-1960 (PMID: 28368536)
Costantino S, Akhmedov A, Melina G, Mohammed SA, Othman A, Ambrosini S, Wijnen WJ, Sada L, Ciavarella GM, Liberale L, Tanner FC, Matter CM, Hornemann T, Volpe M, Mechta-Grigoriou F, Camici GG, Sinatra R, Lüscher TF, Paneni F (2019) Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur Heart J 40(12):997–1008. https://doi.org/10.1093/eurheartj/ehy903 (PMID: 30629164)
Pofi R, Giannetta E, Galea N, Francone M, Campolo F, Barbagallo F, Gianfrilli D, Venneri MA, Filardi T, Cristini C, Antonini G, Badagliacca R, Frati G, Lenzi A, Carbone I, Isidori AM (2021) Diabetic cardiomiopathy progression is triggered by miR122-5p and involves extracellular matrix: a 5-year prospective study. JACC Cardiovasc Imaging 14(6):1130–1142. https://doi.org/10.1016/j.jcmg.2020.10.009 (Epub 2020 Nov 18 PMID: 33221242)
Boussageon R, Bejan-Angoulvant T, SaadatianElahi M et al (2011) Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343:d4169
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57(4):660–671. https://doi.org/10.1007/s00125-014-3171-6
Frustaci A, Kajstura J, Chimenti C et al (2000) Myocardial cell death in human diabetes. Circ Res 87:1123–1132
Boudina S, Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11(1):31–39. https://doi.org/10.1007/s11154-010-9131-7
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60(6):1770–8. https://doi.org/10.2337/db10-0351. Epub 2011 May 11. PMID: 21562078; PMCID: PMC3114402
Lundbaek K (1954) Diabetic angiopathy: a specific vascular disease. Lancet 266(6808):377–379. https://doi.org/10.1016/s0140-6736(54)90924-1 (PMID: 13131862)
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30(6):595–602. https://doi.org/10.1016/0002-9149(72)90595-4 (PMID: 4263660)
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34(1):29–34. https://doi.org/10.1016/0002-9149(74)90089-7 (PMID: 4835750)
Maisch B, Alter P, Pankuweit S (2011) Diabetic cardiomyopathy–fact or fiction? Herz 36(2):102–115. https://doi.org/10.1007/s00059-011-3429-4 (PMID: 21424347)
Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV (2000) Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101(19):2271–2276. https://doi.org/10.1161/01.cir.101.19.2271 (PMID: 10811594)
Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, Clarke K, Rider OJ, Neubauer S (2016) Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65(1):44–52. https://doi.org/10.2337/db15-0627. Epub 2015 Oct 5. PMID: 26438611; PMCID: PMC4890658
MinW, Bin ZW, Quan ZB, Hui ZJ, Sheng FG (2009) The signal transduction pathway of PKC/NF-kappa B/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol 8:8
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323:236–241
Hamdani N, Costantino S, Mügge A, Lebeche D, Tschöpe C, Thum T, Paneni F (2021) Leveraging clinical epigenetics in heart failure with preserved ejection fraction: a call for individualized therapies. Eur Heart J 42(20):1940–1958. https://doi.org/10.1093/eurheartj/ehab197 (PMID: 33948637)
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) Wytyczne ESC dotyczące diagnostyki i leczenia ostrej i przewlekłej niewydolności serca w 2016 roku [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol 74(10):1037–1147. Polish. https://doi.org/10.5603/KP.2016.0141. PMID: 27748494
Park JJ (2021) Epidemiology, pathophysiology, diagnosis and treatment of heart failure in diabetes. Diabetes Metab J 45(2):146–157. https://doi.org/10.4093/dmj.2020.0282. Epub 2021 Mar 25. PMID: 33813813; PMCID: PMC8024162
Paneni F, Costantino S, Hamdani N (2020) Regression of left ventricular hypertrophy with SGLT2 inhibitors. Eur Heart J 41(36):3433–3436. https://doi.org/10.1093/eurheartj/ehaa530 (PMID: 32620974)
Hölscher ME, Bode C, Bugger H (2016) Diabetic cardiomyopathy: does the type of diabetes matter?. Int J Mol Sci 17(12):2136. Published 2016 Dec 18. https://doi.org/10.3390/ijms17122136
Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, Minatoguchi S (2015) Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11(7):1146–1160. https://doi.org/10.1080/15548627.2015.1051295.PMID:26042865;PMCID:PMC4590644
Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED (2008) Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 57(11):2924–32. https://doi.org/10.2337/db08-0079. Epub 2008 Aug 4. PMID: 18678617; PMCID: PMC2570388
Tate M, Deo M, Cao AH, Hood SG, Huynh K, Kiriazis H, Du XJ, Julius TL, Figtree GA, Dusting GJ, Kaye DM, Ritchie RH (2017) Insulin replacement limits progression of diabetic cardiomyopathy in the low-dose streptozotocin-induced diabetic rat. Diab Vasc Dis Res 14(5):423–433. https://doi.org/10.1177/1479164117710390 (Epub 2017 May 31 PMID: 28565941)
Pollack PS, Malhotra A, Fein FS, Scheuer J (1986) Effects of diabetes on cardiac contractile proteins in rabbits and reversal with insulin. Am J Physiol 251(2 Pt 2):H448–H454. https://doi.org/10.1152/ajpheart.1986.251.2.H448 (PMID: 2943166)
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prandi, F.R., Evangelista, I., Sergi, D. et al. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev 28, 597–606 (2023). https://doi.org/10.1007/s10741-021-10200-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10200-y