Abstract
Glycosylation is the most abundant and complex protein modification, and can have a profound structural and functional effect on the conjugate. The oligosaccharide fraction is recognized to be involved in multiple biological processes, and to affect proteins physical properties, and has consequentially been labeled a critical quality attribute of biopharmaceuticals. Additionally, due to recent advances in analytical methods and analysis software, glycosylation is targeted in the search for disease biomarkers for early diagnosis and patient stratification. Biofluids such as saliva, serum or plasma are of great use in this regard, as they are easily accessible and can provide relevant glycosylation information. Thus, as the assessment of protein glycosylation is becoming a major element in clinical and biopharmaceutical research, this review aims to convey the current state of knowledge on the N-glycosylation of the major plasma glycoproteins alpha-1-acid glycoprotein, alpha-1-antitrypsin, alpha-1B-glycoprotein, alpha-2-HS-glycoprotein, alpha-2-macroglobulin, antithrombin-III, apolipoprotein B-100, apolipoprotein D, apolipoprotein F, beta-2-glycoprotein 1, ceruloplasmin, fibrinogen, immunoglobulin (Ig) A, IgG, IgM, haptoglobin, hemopexin, histidine-rich glycoprotein, kininogen-1, serotransferrin, vitronectin, and zinc-alpha-2-glycoprotein. In addition, the less abundant immunoglobulins D and E are included because of their major relevance in immunology and biopharmaceutical research. Where available, the glycosylation is described in a site-specific manner. In the discussion, we put the glycosylation of individual proteins into perspective and speculate how the individual proteins may contribute to a total plasma N-glycosylation profile determined at the released glycan level.
Similar content being viewed by others
Introduction
Protein glycosylation is recognized to be involved in a multitude of biological processes such as receptor interaction, immune response, protein secretion and transport [1–6]. In addition, glycosylation affects protein properties such as solubility, stability and folding [7–10]. A given protein can have multiple sites of glycosylation, and its glycoforms can differ by site occupancy (macroheterogeneity) and occupying glycan structures (microheterogeneity) [11–13]. The biosynthetic pathways leading up to this variety of glycans depend on multiple parameters and can be influenced by many factors including genetic regulation, the availability of nucleotide sugars, the time spent in the endoplasmic reticulum and Golgi apparatus, as well as the accessibility of a particular glycosylation site [10, 14–17].
Protein glycosylation can differ between persons, but is remarkably stable per individual [18]. It is only when the homeostasis of a person changes, by lifestyle or pathological conditions, that the glycosylation will change notably [19]. Large studies comprising thousands of individuals have identified glycosylation to correlate with age, sex and lifestyle [14, 20, 21]. Examples of such changes are the increase in bisection and decrease of galactosylation and sialylation of IgG with age [19, 20, 22–24].
At the same time, specific studies based on smaller sample sets have revealed changes of glycosylation in various diseases, inflammatory states, congenital disorders of glycosylation (CDGs), but also throughout pregnancy where an increase in galactosylation and sialylation, as well as a decrease in bisection was reported [25–28]. In addition, specific glycoforms can be targeted by viruses or bacteria or serve as a pro- or anti-inflammatory signal [29–33]. All of this opens up the possibility to use glycans as an early biomarker for disease or to assist personalized medicine by patient stratification [34, 35].
Recent advances in chromatographic separation, mass spectrometry, robotization and automated data processing allow the rapid analysis of glycosylation, and facilitate the development of novel biomarkers [36–39]. While the glycosylation analysis of an easily obtainable biofluid like plasma can be of considerable interest to a clinical situation, the interpretation of data may be complicated when analyzing a complex protein mixture. For example, when analyzing total plasma N-glycosylation (TPNG) of a clinical cohort at the released glycan level, it is not directly apparent whether an observed change originates from a change in relative protein abundance, in the relative glycoforms of a specific protein, or whether it reflects a general regulatory effect influencing the glycosylation of many different glycoproteins. We expect that a better understanding of the glycosylation of individual proteins of human plasma will help to put total plasma N-glycomic changes into perspective.
As the previous review on plasma protein N-glycosylation originates from 2008 [40], we here strive to convey the current state of knowledge on the subject, including a larger number of proteins. The proteins described in this review were selected based on their plasma levels, additionally including the immunoglobulin family due to its major clinical and biopharmaceutical interest. The 24 glycoproteins covered in this review account for approximately 30 mg/mL of the 70–75 mg/mL of the total plasma protein concentration, thus representing most of the human TPNG (albumin is present at levels of 40 mg/mL but is not glycosylated) [41–43].
Furthermore, we tried to limit our review to human plasma and serum but we also reported findings coming from other biological fluids when information complementary information could be added. The N-glycosylation of the proteins is reported both on a general level and, where available, with site specific information about glycan composition, glycan structure and occupancy. The information is condensed in Table 1, and a schematic representation of the relative protein contribution to each specific glycan composition is reported in Fig. 1.
Schematic representation of the relative protein contribution to each specific glycan composition. To obtain these numbers, the contribution of a glycan composition to the total glycan pool of a given protein was multiplied by the abundance of that protein as well as the number of glycosylation sites confirmed to be occupied. Protein concentrations were taken from large studies when available and a mean value was calculated from the reported ranges otherwise. The molecular mass used is as reported by SDS-PAGE for the glycoproteins or calculated from the phenotype distribution for haptoglobin. The general Oxford notation was used for naming the glycan structures. For details on the calculation see Supplementary Table 1
Throughout the text, Oxford nomenclature has been used to annotate individual glycan structures or compositions with A giving the number of antennae, F for the fucose (location specific), B for bisecting N-acetylglucosamine, G for galactoses and S for sialic acids. The number directly after the letter indicates the quantity of the specific features and the number in parenthesis its linkage. UniProt numbering was used for sequence and site identification.
Alpha-1-acid glycoprotein (P02763; P19652)
Alpha-1-acid glycoprotein (AGP), also known as orosmucoid-1, is a 201 amino acid glycoprotein, which includes an 18 amino acid signal peptide. The molecular weight of the bare protein is 23.5 kDa, but the carbohydrate content leads to observed masses around 41–43 kDa [44]. Two isoforms are found in plasma (AGP1 and AGP2 encoded by ORM1 and ORM2 respectively), differing in 22 amino acids [44]. The protein is expressed by the liver and secreted in a monomeric form into the circulation, where it is observed in concentrations between 0.36 and 1.46 mg/mL with a mean of 0.77 mg/mL, men having slightly higher levels than women [204, 205]. The concentration of AGP has been reported to increase with age in females but not in males. Being an acute phase protein, its serum concentration rises in response to inflammatory stimuli, potentially increasing the concentration two- to four-fold [205].
The main functions of AGP are acute phase negative modulation of the complement system and transport of lipophilic compounds, both of these heavily modulated by the glycosylation of the protein [206, 207]. The immunomodulatory function is expected to be via interaction with selectins at a given site of injury (with sialyl-Lewis X as ligand), and inhibiting local complement deposition by charge and receptor competition [207]. As AGP may be used to transport lipophilic and acidic drugs to a site of injury, it is regarded as a good target for therapeutic development [206].
Glycosylation
AGP has five N-linked glycosylation sites, namely Asn33, Asn56, Asn72, Asn93 and Asn103. Overall, the glycosylation was determined to mainly consist of fully sialylated tri- and tetraantennary structures, with potential antennary fucosylation in the form of sialyl-Lewis X [44, 45]. Site specific glycosylation was determined by high-performance (HP) liquid chromatography (LC)-electrospray ionization (ESI)-mass spectrometry (MS) and matrix assisted laser desorption/ionization (MALDI)-time-of-flight (TOF)-MS of tryptic glycopeptides [46]. Asn33 mainly contains the triantennary structure A3G3S3 (60 %) together with its antennary fucosylated variant A3FG3S3 (20 %), as well as some non-complete sialylated glycoforms (A3G3S2, 12.5 %). Asn56 contains similar structures (A3G3S3, 55 %; A3FG3S3, 12.5 %; A3G3S2, 10 %) and a fraction of diantennary glycans (A2G2S2, 22.5 %). Asn72 is occupied by a higher level of antennarity, having, next to its triantennary glycans (A3G3S3, 15 %), a number of tetraantennary compositions (A4G4S4, 30 %; A4G4S3, 15 %; A4G4S2, 10 %; A4FG4S3, 5 %; A4FG4S4, 5 %). A similar situation is seen at Asn93 (A4G4S4, 22.5 %; A4G4S3, 20 %; A3G3S3, 17.5 %; A4FG4S4, 7.5 %; A4FG4S3, 7.5 %; A3FG3S3, 7.5 %; A4G4S2, 7.5 %) and Asn103 (A4G4S4, 45 %; A3G3S3, 20 %; A4G4S2, 10 %; A4G4S3, 7.5 %; A3FG3S3, 5 %) [45, 46] (Table 1).
The glycosylation of AGP changes considerably with varying conditions. For instance, during the early stages of an acute-phase immune response the levels of fucosylated glycans (sialyl-Lewis X) increase significantly [45, 47–49], which continues to increase throughout the acute phase immune response [50]. In rheumatoid arthritis both fucosylation and sialylation have shown to increase significantly [51].
Alpha-1-antitrypsin (P01009)
Alpha-1-antitrypsin (AAT), also known as alpha-1-protease inhibitor, alpha-1-antiproteinase or serpin A1, consists of 418 amino acids (including a 24 amino acid signal peptide) with an apparent mass of 51 kDa (including glycosylation). It is mainly produced in the liver by hepatocytes, but is also synthesized in monocytes, intestinal epithelial cells, and in the cornea [52, 208–211]. Due to its small size and polar properties, the glycoprotein can easily move into tissue fluids [52]. In healthy individuals, a plasma level of approximately 1.1 mg/mL is found, but the concentration can increase three- to four-fold during inflammation [212–215]. AAT occurs as three different amino acid sequences, of which the first is set as the standard sequence. Form 2 differs in the amino acid sequence 356–418 and form 3 lacks the amino acid sequence 307–418.
AAT inhibits a wide range of serine proteases, protecting tissues from enzymatic attacks [216]. Neutrophil elastase is its prime target, thereby preventing proteolytic destruction of elastase in the tissue of the lower respiratory tract (emphysema) [217]. It has been shown that AAT has anti-inflammatory properties and therefore it could potentially be used as a therapeutic agent for rheumatoid arthritis and type 1 diabetes [218, 219].
Glycosylation
Three N-glycosylation sites have been identified on AAT, located at Asn70, Asn107 and Asn271 [52–54]. MALDI-TOF-MS analysis on released glycans revealed mainly di- and triantennary complex type species. Isoelectric focusing furthermore revealed eight different charge isoforms of AAT, of which isoform 4 (M4) and isoform 6 (M6) were the most abundant ones. Of M4, the most pronounced glycans were diantennary disialylated (A2G2S2) and triantennary trisialylated (A3G3S3) with a ratio of 2:1. Isoform M6 was mainly occupied with A2G2S2 structures [55].
LC-MS/MS analysis on tryptic glycopeptides treated with various specific exoglycosidases enabled a precise determination of the glycosylation in a site-specific manner [54]. Asn70 and Asn271 mainly contain diantennary disialylated (A2(2)G2(4)S2(6)) structures (91.3 and 99.3 % respectively), while core fucosylation (F(6)A2(2)G2(4)S2(6)) is less abundant (8.6 and 0.7 % respectively). Asn107 shows the highest variability of the sites, containing diantennary disialylated species A2(2)G2(4)S2(6), 52.5 %, with possible core fucosylation F(6)A2(2)G2(4)S2(6), 1.5 %), and 29.5 % triantennary trisialylated species A3(2,4,2)G3(4)S3(6,3,6)) with possible antennary fucosylation (A3(2,4,2)F(3)G3(4)S3(6,3,6), 16.7 % (Table 1). In addition, a small fraction of Asn107 is tetraantennary fully sialylated with potential antennary fucosylation. Interestingly, the diantennary structures contained mainly (α1-6-linked) core-fucosylation, while on triantennary structures the fucose was mainly detected as sialyl-Lewis X on the β1-4-linked N-acetylglucosamine of the α1-3-arm [54].
In a non-site-specific study, the glycosylation of AAT has been associated with physiological parameters such as BMI, cholesterol, glucose and insulin level. The same study showed that the changes in glycosylation could be found related to age and sex [56]. Furthermore, additional AAT isoelectric isoforms were identified in CDG-I (CDG-Ia and CDG-Ic) i.e. non-, mono- and diglycosylation across the three sites. A clear pattern could be found for which sites were occupied, as only Asn70 was occupied in the monoglycosylated form, and Asn70 and Asn271 were occupied in the diglycosylated isoform [57].
Alpha-1B-glycoprotein (P04217)
Alpha-1B-glycoprotein (A1BG) is a 474 amino acid polypeptide with an apparent mass of 63 kDa (including glycosylation) [58]. The protein consists of five repetitive domains that show high homology with known immunoglobulin heavy and light chain variable domains, making the protein part of the immunoglobulin superfamily. A1BG is mainly produced in the liver, and is secreted to plasma to levels of approximately 0.22 mg/mL [58, 220]. The overall function of the protein is still unknown, but it has been found to bind cysteine-rich secretory protein 3 (CRISP3) [221], and has been associated with breast, liver, pancreas and bladder cancer, as well as with steroid-resistant nephrotic syndrome [222–226]. In addition, it has recently been proposed as an autoantigen in rheumatoid arthritis [227].
Glycosylation
In A1BG, the N-glycosylation consensus motif (Asn-X-Ser/Thr) has been found at four locations Asn44, Asn179, Asn363, and Asn371 [58]. The occupancy of these sites has been verified by deglycosylated peptide LC-MS(/MS) and LC-Fourier transform ion cyclotron resonance (FTICR)-MS, but degrees of occupancy remain unknown [59, 60]. In addition, overall or site-specific glycosylation analysis also has not been performed for A1BG as of yet, although one source reports blood derived high-density lipoprotein (HDL)-associated A1BG Asn363 to be (at least) glycosylated with diantennary nonfucosylated monosialylated species [61]. Also, while not necessarily predictive for plasma glycoprotein glycosylation, in cerebrospinal fluid (CSF) Asn44 was shown to contain nonfucosylated di- (96 %) and triantennary (4 %) structures with at least one sialic acid [62]. Little is known about the changes in glycosylation of A1BG with disease.
Alpha-2-HS-glycoprotein (P02765)
Alpha-2-HS-glycoprotein (A2HSG), also known as fetuin-A, alpha-2-Z-globulin, ba-alpha-2-glycoprotein and alpha-2-Heremans-Schmid-glycoprotein, is a 367 amino acids (18 amino acid signal peptide), 51–67 kDa glycoprotein [63, 64, 228]. It is built up from an A-chain (282 amino acids) and B-chain (27 amino acids) with a linker sequence (40 amino acids) [135, 229]. Originating from the liver, the protein is found at plasma levels of 0.3–0.6 mg/mL [229]. A2HSG acts at several sites and in a wide variety of (patho)physiological processes in the human system. Prominent functions include the scavenging of phosphate and free calcium, thereby preventing calcification, as well as binding and protecting matrix metalloproteases. In addition, the protein is known to bind the insulin receptor [230–233].
Increased levels of A2HSG are associated with obesity and type 2 diabetes mellitus [234]. On the other hand, decreased levels of A2HSG are found to cause several negative growth effects [230]. Furthermore, the protein has shown to protect a fetus from the maternal immune system by inhibition of tumor necrosis factor [231, 235]
Glycosylation
The A-chain of A2HSG contains two N-glycosylation sites at Asn156 and Asn176, as well as two O-glycosylation sites at Thr256 and Thr270 [63]. The B-chain contains one core 1 O-glycan on Ser346, and no N-glycans [64]. Exoglycosidase treatment has reported 6.2 sialic acids to be present per A2HSG molecule, of which 2.5 are α2-3-linked and 3.7 are α2-6-linked [65]. Sequentially, four galactoses in β1-4-linkage were released from N-acetylglucosamines, pointing towards two diantennary N-glycans in addition to the O-glycosylation [65]. LC-ESI-MS experiments have confirmed these findings, reporting around 96 % A2G2S2 glycosylation to be present on A2HSG [66]. Furthermore, low levels of fucosylated glycans were observed, at least on Asn156 [66, 67].
Differential abundance of Asn156 glycopeptides has been shown in pancreatic cancer and pancreatitis, with increased levels of fully sialylated triantennary glycans with or without fucose, and a decrease in the A2G2S2 structure in pancreatitis [68].
Alpha-2-macroglobulin (P01023)
Alpha-2-macroglobulin (alpha2M), also known as C3 or PZP-like alpha-2-macroglobulin domain-containing protein 5, is a 1474 amino acid (23 amino acid signal peptide) 720 kDa (glycosylated) glycoprotein consisting of four similar 180 kDa subunits (160 kDa without glycosylation) which are linked by disulfide bridges [69]. It is produced by the liver and present at plasma levels of approximately 1.2 mg/mL [236]. The main function of alpha2M is to bait and trap proteinases [69]. To do this, the protein contains a bait peptide sequence known to interact with many common plasma proteases such as trypsin, chymotrypsin, and various others in the complement system. Upon proteolysis, a conformation change in alpha2M traps the causative protease and the complex is subsequently cleared from the plasma [237–239].
Glycosylation
Eight N-glycosylation sites have been identified on each alpha2M subunit at Asn55, Asn70, Asn247, Asn396, Asn410, Asn869, Asn991 and Asn1424 [59, 60, 67, 69–71]. The total pool of alpha2M-derived glycans was analyzed by LC-fluorescence with exoglycosidase digestion. This revealed a high abundance of diantennary structures, both non-fucosylated (55 %) and core-fucosylated (30 %), which are mainly mono- and disialylated (A2G2S2, A2G2S1, FA2G2S2, FA2G2S2) [72]. In addition, Man5-7 type structures was detected as well (8 %) as species with a lower degree of galactosylation and sialylation. Low levels of triantennary structures have also been identified [72].
Interestingly, the high-mannose type glycans have been shown to specifically occur at Asn869 with a relative abundance of approximately 70 %, the other 30 % being FA1G1S1 [72]. This high-mannose type glycosylation is likely the means by which alpha2M interacts with mannose-binding lectin (MBL) to target proteases present on the surface of invading microorganisms [72]. The Asn869 occupancy ratio suggests that each alpha2M tetramer contains three oligomannose glycosylated Asn869 sites and one FA1G1S1, although this is speculative [72]. The other N-glycosylation sites mainly contain complex type glycans and glycoproteomic analysis suggests that the core-fucosylated species are present to at least some degree at specific sites Asn55 and Asn1424 [67].
Changes in the glycosylation of alpha2M have been associated with autoimmune diseases and cancer. Site occupancy in particular has been linked with systemic lupus erythematosus, while a compositional change has been described in multiple sclerosis [73, 74].
Antithrombin-III (P01008)
Antithrombin-III (AT-III, generally referred to as antithrombin), encoded by the SERPINC1 gene, is a single chain 464 amino acid (32 amino acid signal peptide) protein of approximately 58 kDa, of which 17 % are carbohydrates [240–242]. It is part of the serine protease inhibitor family. The concentration of antithrombin in blood was found to be 0.15 mg/mL [243]. The protein can be found in an α and β form which differ in the number of occupied glycosylation sites and of which α is 10–20 times more abundant [75]. Antithrombin participates in the regulation of blood coagulation by inactivating thrombin, factor IXa, Xa, XIa, XIIa, and other serine proteases [244]. Its function is enhanced by heparin and heparan sulfate [76, 245, 246]. Several thrombosis disorders are associated with antithrombin deficiency (ATD), both inherited and acquired. Type I ATD shows reduced concentrations of antithrombin, while type II ATD generally shows normal concentrations with reduced heparin binding and thus lower functionality [247].
Glycosylation
The sequence of antithrombin shows four potential N-glycosylation sites: Asn128, Asn167, Asn187 and Asn224. The α form is fully glycosylated, while the β form is not glycosylated at Asn167 [77, 78]. The β form binds heparin more efficiently and thus shows an enhanced anticoagulant effect. Several studies suggest that the Asn-X-Thr motif of Asn128, Asn187 and Asn224 are in general glycosylated more easily than the Asn-X-Ser motif of Asn167 [79–81].
The glycans present on AT-III are mainly of the diantennary complex type without core fucose, bearing one (0–30 %) or two (70–100 %) α2-6-linked sialic acids, as it was established using chemical and enzymatic methods [82, 83]. Using MALDI and LC-ESI-MS these findings have been confirmed in a site-specific manner [75, 76, 84, 85]. β-AT was exclusively decorated with three diantennary fully sialylated structures (A2G2S2, 4.2 %), with trace amounts of core fucose on one of the glycan (FA2G2S2, 1.3 %). At Asn128 and Asn224 of α-AT, only the A2G2S1 and A2G2S2 structures were identified. At Asn167, occupied only in the α form of AT-III, A3G3S3 has additionally been detected, whereas Asn187 showed the most variability, bearing also a minor amount of fucose (FA2G2S2). Furthermore, at Asn187 some A3G3S2 has been observed. All glycoforms other than A2G2S2 are mentioned to be minor, although no relative or absolute quantification has been performed [75, 76, 84, 85].
A mutation associated with type II antithrombin deficiency (K241E), although not adjacent to a glycosylation consensus sequence, was found to result in decreased heparin binding due to the presence of core fucose [86].
Apolipoprotein B-100 (P04114)
Apolipoprotein (Apo) B-100 is a 550 kDa 4560 amino acid protein (4536 amino acids without the signal peptide, corresponding to a theoretical mass of 513 kDa without glycosylation) found in low density and very low density lipoproteins (LDL and VLDL) [248, 249]. A shorter isoform found in chylomicrons, named Apo B-48, is coded by the same gene, but contains only 48 % of Apo B-100 sequence [250, 251]. Apo B-100 is exclusively synthetized by the liver, while Apo B-48 is synthetized in the small intestine [252]. Apo B-100 is found in plasma at concentrations of approximately 0.5 mg/mL (0.88 to 0.97 mmol) [34, 212, 253, 254]. The protein has a major role in the assembly of VLDL and lipoproteins, and transports the majority of plasma cholesterol [255–257]. It can be covalently linked to Apo A to form the lipoprotein(a) particle. Apo A itself is a low abundant plasma glycoprotein possessing one N-glycosylation site located at Asn263 (mainly occupied by diantennary mono- and disialylated N-glycans) [87, 258].
In coronary heart disease, the ratio of LDL-Apo A/B-100 is used for estimating the risk of acute myocardial infarction [253]. Apo B-100 and Apo B-48 mutations caused by APOB100 and MTP (microsomal triglyceride transfer protein) gene defects are associated with metabolic disorders like abetalipoproteinaemia, hypobetalipoproteinemia and hypercholesterolemia [259–261].
Glycosylation
Apo B-100 is highly glycosylated, and contains 19 potential N-glycosylation sites located at Asn34, Asn185, Asn983, Asn1368, Asn1377, Asn1523, Asn2239, Asn2560, Asn2779, Asn2982, Asn3101, Asn3224, Asn3336, Asn3358, Asn3411, Asn3465, Asn3895, Asn4237 and Asn4431. Of these, 17 are reported occupied by diantennary complex type glycans, as well as by high mannose and hybrid type structures [11, 87]. LC-fluorescence with exoglycosidase digestion has revealed the major glycans to be A2G2S1(6) (29.2 %), A2G2S2(6) (23.6 %), A2G2 (7.2 %), Man9 (8.6 %) and Man5 (6.9 %) [87]. In addition, low levels of the Man6-8 have been reported. Most of the sialic acids were α2-6-linked (91 %) [87].
A site specific analysis of the glycosylation has been performed by LC-ESI-MS(/MS) on tryptic and chymotryptic glycopeptides [11]. It was shown that the high mannose type glycans were mainly present on sites Asn185, Asn1368, Asn1377, Asn3336 and Asn3358, while the complex type (mono- and disialylated diantennary) glycans were located at Asn983, Asn2239, Asn2779, Asn2982, Asn3101, Asn3224, Asn3465, Asn3895, Asn4237 and Asn4431. Ans3895 is exceptional in this regard, as triantennary compositions have been observed as well. The largest variation is present on sites Asn1523 and Asn3411, as these display oligomannose, hybrid and complex structures. Asn3411, the nearest N-glycosylation site to the receptor of the LDL-binding site shows degrees of fucosylation. Asn34 and Asn2560 are not reported to be glycosylated [11].
The role of the glycans structures in LDL and/or Apo B-100 has been examined in several studies but their exact function is still unknown, although its degree of sialylation might serve the atherogenic properties of LDLs [87–90].
Apolipoprotein D (P05090)
Apolipoprotein D (Apo D), also referred to as thin line polypeptide, is a small glycoprotein of 189 amino acids (with a signal peptide of 20 amino acids), with a molecular weight varying between 19 and 32 kDa depending on its glycosylation [262, 263]. While it shares their name, it does in fact not resemble other apolipoproteins, and shares more homology with the lipocalin protein family [264]. It was originally assimilated to the apolipoprotein family due to its early association with lipid transport. Apo D is mainly synthetized in fibroblasts and to a lesser extent in the liver and intestine, where the other apolipoproteins are usually produced [265]. Its plasma levels are approximately 0.1 mg/mL [266, 267]. The common form of Apo D in plasma is a monomer, although it can also exist as a heterodimer linked to apolipoprotein A-2 via a disulfide bridge.
Apo D can form complexes with lecithin cholesterol acyltransferase and is implicated in the transport and transformation of lipids [264, 268–270]. It has been reported to have a potential role in colorectal cancer [265]. In addition, the protein is present at high concentrations in the cyst fluid where its concentration can be 500 times higher than in plasma, which can be associated with an increased risk of breast cancer [271–273]. Apo D has a tendency to accumulate in CSF and peripheral nerves of patients with Alzheimer’s disease and other neurodegenerative conditions [274, 275]. A positive correlation between age and Apo D levels has been reported in females, but not in men [276, 277].
Glycosylation
Two glycosylation sites have been reported and confirmed for Apo D, namely Asn65 and Asn98 [59, 60, 91]. These are mainly occupied by complex type N-glycans ranging from diantennary to tetraantennary structures, with potential elongation of the antennae in the form of N-acetyllactosamine (LacNAc) repeats [91]. LC-MS with exoglycosidase digestion has revealed the most abundant glycoforms per site as well. Asn65 mainly contains nonfucosylated triantennary structures with full sialylation (A3G3S3), less abundant signals including di- and tetraantennary species with high degrees of sialylation (A2G2S2; A4G4S4). Contrarily, Asn98 predominantly contains fucosylated species, also ranging from di- to tetraantennary, here the main signal being diantennary (A2FG2S2) [62, 67, 91, 92]. Treatment with β-galactosidase failed to trim one antenna of its galactosylation, strongly suggesting the presence of the antennary fucosylation, known to prevent this digestion [91, 93]. Studies have shown the implication of Apo D in conditions like Alzheimer’s disease but no information about the role of glycosylation has been reported yet.
Apolipoprotein F (Q13790)
Apolipoprotein F (Apo F), also called lipid transfer inhibitor protein (LTIP), is a glycoprotein with an apparent mass of 29 kDa. The polypeptide chain of 326 amino acids is processed, with the first 165 amino acids being the signal peptide and the propeptide, resulting in a theoretical mass of 17.4 kDa for the peptide backbone of the mature protein [278]. Apo F is expressed in the liver and is secreted in plasma to concentrations of 0.07 mg/mL in females and 0.10 mg/mL in males [94, 278, 279]. The protein regulates cholesterol transport, inhibits cholesteryl ester transfer protein (CETP), and is found in combination with lipoproteins of all subclasses (high density, low density and very low density lipoproteins (HDL, LDL, VLDL)) as well as with apolipoproteins A1 and A2 [279, 280].
Glycosylation
Three potential N-glycosylation sites of Apo F are located at Asn118, Asn139 and Asn267, as well as one O-glycosylation site at Thr291 [94, 95]. Asn118 and Asn139 are glycosylated with high-mannose structures, as proven by exoglycosidase treatment, but will not contribute to plasma glycosylation as they are part of the proprotein [95]. Asn267, on the other hand, is not sensitive to this treatment, suggesting that it would contain complex-type N-glycans [95]. The presence of sialic acids on the protein has been indicated by sialidase treatment with western blotting as readout [94]. However, as O-glycanase has also shown the presence of O-glycans on the protein, it is unclear whether the sialylation arises from the N- or O-glycosylation [94]. In CSF, the O-glycans on Thr291 are reported to be of core 1 or 8 type [96]. No disease-related information is available with regard to the glycosylation of Apo F.
Beta-2-glycoprotein 1 (P02749)
Beta-2-glycoprotein 1 (B2GPI) is also called apolipoprotein H, APC inhibitor, activated protein C-binding protein, and anticardiolipin cofactor. It is a 50 kDa (including around 19 % carbohydrate content) 345 amino acid single polypeptide chain (with a signal peptide of 19 amino acid) belonging to the complement control protein (CCP) superfamily [97]. It consists of five similar CCP domains of approximately 60 amino acids [281]. B2GPI is mostly synthetized in hepatocytes and is found in blood at around 0.2 mg/mL [282]. The main function of B2GPI is the scavenging of negatively charged compounds such as DNA, sialylated glycoproteins, and (phospho)lipids, which may otherwise induce unwanted coagulation and platelet aggregation [283–285]. The precise binding properties of the protein depend on the conformation, i.e. open or closed, which is proposed to be dependent on the glycosylation [98, 99, 286].
The serum level of B2GPI increases with age, and is reduced during pregnancy and for patients suffering from stroke and myocardial infarctions [98, 287]. Additionally, it is the major antigen in antiphospholipid syndrome [98, 99].
Glycosylation
B2GPI possesses four theoretical N-glycosylation sites at Asn162, Asn183, Asn193 and Asn253, as well as an O-glycosylation site at Thr149 [97, 98, 100, 101]. The N-glycosylation sites have been confirmed by crystallography (finding attached N-acetylglucosamines and mannoses) as well as by deglycosylated Lys-C peptide reverse phase (RP)-LC-MS after lectin capture [59, 101]. Generally, the glycosylation of B2GPI is of the di- and triantennary type containing high levels of sialylation, with minor amounts of fucosylation [99]. Site-specific information is available only for Asn162 and Asn193 [99].
Glycopeptide LC-ESI-quadrupole (Q)-TOF-MS revealed the glycosylation of Asn162 to be 67 % diantennary disialylated (A2G2S2) and 22 % triantennary trisialylated (A3G3S3), minor species including the di- and triantennary species lacking one sialic acid (5 and 3 % respectively) [99]. The Asn193 site showed the same compositions, but with a higher level of triantennary species (35 %) and a corresponding lower percentage of diantennary species (49 %). Minor species again include the incompletely sialylated variants (8 and 7 % for the di- and triantennary species respectively) [99]. The findings were confirmed by MALDI-QTOF-MS, although a lower degree of sialylation was observed. This difference is likely due to the tendency of MALDI ionization to induce in-source and metastable decay of sialylated glycan species [99, 102]. For the two noncharacterized N-glycosylation sites (Asn183 and Asn253) di- and triantennary glycans are expected as well, given the 19 % of the total protein weight being attributed to the carbohydrate content [101].
Patients suffering from antiphospholipid syndrome (APS) showed a decrease in the amount triantennary sialylated glycans, and thus a relative increase in diantennary fully sialylated ones. This effect was particularly pronounced for Asn162 [99].
Ceruloplasmin (P00450)
Ceruloplasmin (CP), also called ferroxidase, is a 132 kDa (120 kDa without glycosylation) 1065 amino acid (19 of which are signal peptide) glycoprotein synthesized by the liver [103]. It consists of a single polypeptide chain, and belongs to the multicopper oxidase family [103]. Concentrations for CP range from 0.15 to 0.96 mg/mL with a mean of 0.36 mg/mL, while elevated levels have been reported upon inflammatory stimulation [34, 288, 289]. CP can bind six to seven atoms of copper, in this manner containing and transporting 95 % of the copper found in plasma. The main function of the protein, however, is in iron metabolism. CP has ferroxidase activity oxidizing Fe2+ to Fe3+ without releasing radical oxygen species, while also facilitating iron transport across the cell membrane [103].
Glycosylation
Of the seven potential CP N-glycosylation sites Asn138, Asn227, Asn358, Asn397, Asn588, Asn762 and Asn926, four (Asn138, Asn358, Asn397, and Asn762) are confirmed to be glycosylated [59]. The remaining sites (Asn227, Asn588, and Asn926) are all in a β-strand within a hydrophobic region, potentially preventing site occupation [103]. NMR spectroscopy has revealed the overall CP glycan species to be sialylated diantennary A2(2)G2(4)S2(6) and sialylated triantennary A2(2,2,4)G4(4)S3(6,6,3/6). Partial core fucosylation has been found for the diantennary species, while of the triantennary species the α2-3-linked sialic acid-containing arm can be α1-3-fucosylated to form sialyl-Lewis X [104].
For the confirmed N-glycosylation sites, tryptic glycopeptide LC-ESI-MS(/MS) was used to study the site-specific glycosylation on a compositional level, and relatively similar ratios of di- and triantennary glycan species were found across the sites [105]. Asn138 is mainly occupied by the diantennary structures A2G2S2 (49 %) and FA2G2S2 (26 %), followed by the triantennary structures A3G3S3 (12 %) and A3FG3S3 (10 %). Small amounts of difucosylated species have been detected as well (FA3FG3S3, 3 %). Asn358 contains a higher abundance of diantennary species (A2G2S2 83 %, and FA2G2S2 12 %), the triantennary species A3G3S3 only accounting for 5 %. For Asn397 the main glycan is A2G2S2 (73 %), followed by A3G3S3 (17 %), A3FG3S3 (6 %) and FA2G2S2 (4 %). Analysis of Asn762 showed the main glycan to be A2G2S2 as well (46 %), with the additional compositions A3G3S3 (20 %), FA2G2S2 (16 %), A3FG3S3 (13 %), FA3FG3S3 (2 %), A4G4S4 (1 %) and A4FG4S4 (1 %) [105]. No information about the glycosylation of human ceruloplasmin in disease was found with the preparation of this review.
Fibrinogen (P02671; P02675; P02679)
Fibrinogen is a 340 kDa glycoprotein that is synthesized in the liver by hepatocytes, and plays a key role in blood clotting [290, 291]. The protein consists of two sets of three different polypeptide chains named the α-chain (610 amino acids), β-chain (461 amino acids), and γ-chain (411 amino acids), arranged in a α2β2γ2 hexamer linked by disulfide bonds [106, 292, 293]. In plasma, fibrinogen is typically found at concentrations of 2–6 mg/mL with a mean of 3 mg/mL, with women having slightly higher levels, and it is also present in platelets, lymph nodes, and interstitial fluid [106, 293–296].
Fibrinogen is cleaved by thrombin into fibrin, one of the essential components of blood clots after injury [106, 291, 297]. Furthermore, it acts as a cofactor in platelet aggregation, assists rebuilding of epithelium, and can protect against infections in interferon γ (IFNγ)-mediated hemorrhage [106, 298, 299]. In addition, the protein can facilitate the immune response via the innate and T-cell pathways [300–303].
Glycosylation
The α-chain of fibrinogen is not N-glycosylated, even though it harbors two potential N-glycosylation sites at Asn453 and Asn686. The β- and γ-chain are N-glycosylated at Asn394 and Asn78, respectively [106–108]. By MALDI-TOF-MS and HPLC with exoglycosidase digestion, the predominant glycan structures present on these chains were found to be A2G2S1 (53 %) and A2G2S2 (33 %). Sialic acids are mainly α2-6-linked, but a degree of α2-3-linkage has been reported as well depending on the source or analytical method [109, 110]. Bisecting N-acetylglucosamine and core fucosylation are found in minor quantities [110]. Comparisons between plasma and serum N-glycan profiles revealed that fibrinogen could contribute for 22 % to the total intensity of the diantennary monosialylated structures (A2G2S1) [110].
Site-specific analysis showed diantennary glycans with zero, one or two sialic acids on Asn394 (β-chain) and Asn78 (γ-chain) [107]. The glycosylation sites have been confirmed in studies at the level of deglycosylated glycopeptides, showing occupancy of Asn394 of the β-chain and Asn78 of the γ-chain, and surprisingly on the α-chain Asn686 as well [59, 60, 70, 108]. The β-chain glycosylation site has furthermore been observed in a core-fucose targeted study [67]. In addition to N-glycosylation, all fibrinogen chains may carry O-glycans [107].
The general degree of sialylation may be influencing the solubility of fibrinogen, and thereby play a crucial role in blood clotting processes resulting in different fiber structures. [111–115]. In the Asahi mutant of the γ-chain, Asn334 has been reported to contain an additional N-glycosylation site [116]. Patients exhibiting the Asahi variant of fibrinogen displayed abnormally long blood clothing time, suggesting that the effect induced by that extra glycosylation site disturbs the fibrin polymerization process [116, 117].
Haptoglobin (P00738)
Haptoglobin (Hp) is a 406 amino acid (18 amino acid signal peptide) acute-phase glycoprotein with a peptide backbone of 45 kDa. It is synthesized in the liver by hepatocytes as a single polypeptide chain and is also found in skin [304, 305]. During its synthesis, Hp is cleaved into a light α chain and a heavy β chain that are connected via disulfide bonds. Two variants of the α chain originating from the sequence Val19-Gln160 and differing by the subsequence Glu38-Pro96 can exist, α1 having this subsequence once while α2 has it twice, resulting in α chains of 83 or 142 amino acids with a respective molecular mass of 9 and 16 kDa. The 40 kDa β chain is made of 245 amino acids originating from the sequence Ile162-Asn406 [306, 307]. The combination of different allelic variants of the α chain (α1 and α2) with β chain(s) creates the polymorphism observed in Hp. There are three major Hp phenotypes called Hp1-1, Hp2-1 and Hp2-2. They have a configuration of (α1β)2, (α1β)2 + (α2β)n = 0, 1, 2, … and (α2β)n = 3, 4, 5, …, respectively, which are observed at different ratios among ethnicities [118, 308–310]. Caucasians have around 13 % of phenotype Hp1-1, 46 % of Hp2-1 and 41 % of Hp2-2. Hp is typically found at a plasma levels in the range of 0.6–2.3 mg/mL with a mean of 1.32 mg/mL [118]. Elevated Hp levels have been reported with inflammation and malignant diseases [308, 311, 312]. It should be taken into account that the concentration as well as the molecular mass including glycosylation may vary among phenotypes (86–900 kDa) [118]. The half-life of Hp is found to be on average four days.
The major function of Hp is to protect tissues from oxidative damage by capturing hemoglobin [307, 313]. It has been reported that Hp polymorphism has an effect on its physiological properties, for instance Hp1-1 binds hemoglobin stronger than Hp2-2 [314]. Certain diseases seem to be dependent on the polymorphism, as individuals with the Hp1-1 phenotype seem to have a higher concentration of induced antibodies in their plasma after vaccination, infections or liver diseases compared to the other phenotypes [118, 310].
Glycosylation
Four N-glycosylation sites have been identified on the β-chain of Hp, located at Asn184, Asn207, Asn211 and Asn241 [119–121]. Analysis with (nano-)RPLC-ESI-MS/MS and MALDI-MS/MS of Hp glycopeptides (trypsin and GluC) revealed that all sites are occupied by complex type N-glycans [119, 120]. The site occupancy for Asn184 was determined at 97.7 %, Asn207 at 97.4 %, Asn211 at 98.5 % and Asn241 had a site occupancy of 95.8 %. Treatment with α2-3-sialidase showed that the sialic acids were mainly α2-6-linked, while β1-4-galactosidase treatment revealed that only antennary fucosylation was present, which was in agreement with the obtained collision-induced dissociation (CID) fragmentation spectra [120].
Two recent studies showed some discrepancies in the relative abundances for the identified sites. For example, Asn184 was found to contain mainly diantennary species with two sialic acids (A2G2S2, 88 and 46 %), followed by diantennary monosialylated (A2G2S1, 7 and 38 %) and triantennary disialylated (A3G3S2, 4 and 3 %) glycans. A low percentage of fucosylation was identified (A3FG2S2, 1 and 3 %; A3FG3S2, 0.3 and 1 %) [119, 120].
A possible reason for discrepancies at Asn207/Asn211 is that the first study did not differentiate the two N-glycosylation sites (Asn207 and Asn211) on the same peptide backbone that showed 7 different combinations. The major combinations were 1) one diantennary fully sialylated (A2G2S2) and one triantennary disialylated (A3G3S2, 45 %), 2) two diantennary disialylated glycans (A2G2S2, 30 %), and 3) one diantennary fully sialylated (A2G2S2) and one triantennary disialylated and fucosylated (A3FG3S2, 12 %). The combination of a diantennary monosialylated (A2G2S1) with a diantennary disialylated glycan (A2G2S2) accounted for 6 %, the diantennary and triantennary fully sialylated species (A2G2S2 and A3G3S3) for 5 %, and the remaining combinations accounted for approximately 1 % in total [119]. The second study reported the glycoforms for each site separately due to an additional GluC protease treatment. Asn207 seems to contain mainly A2G2S2 (47 %) and A2G2S1 (39 %), followed by A3G3S1 (7 %) next to some minor tetraantennary and fucosylated species. Interestingly, glycosylation site Asn211 appears to have a higher degree of triantennary species, with A2G2S2 (40 %), A3G3S3 (29 %), A3FG3S3 (21 %), and A3G3S2 (10 %) [120].
The two studies report that Asn241 carries mainly diantennary glycans, A2G2S2 being the most abundant variant with 87 and 47 % (values reported in the two separate studies), followed by A2G2S1 (4 and 26 %), A3G3S1 (n.d. and 10 %), A3G3S2 (6 and 8 %), A3G3S3 (n.d. and 4 %) and A2FG2S1 (<1 and 2 %). Low levels of tetraantennary species have been detected as well, with and without fucosylation varying from mono- to tetrasialylated [119, 120].
Both studies evaluated the glycosylation of Hp in patients with liver cirrhosis (LCH) and hepatocellular carcinoma (HCC). No difference in site occupancy could be observed between healthy and disease, but the number of detected glycoforms was increased (healthy 34 glycoforms, LCH 56 glycoforms, HCC 62 glycoforms) [120]. Increased branching and fucosylation were reported, with species carrying up to five fucoses [119, 121]. Furthermore an increase in sialylation was noticeable for the glycopeptide containing N-glycosylation sites Asn207 and Asn211 [119]. Those carbohydrate structures have been reported in another study along with some new ones but they were not quantified [13].
Furthermore, core-fucosylation was identified on the N-glycosylation site Asn184 [122]. Diantennary disialylated structures contained core-fucosylation (FA2G2S2) instead of antennary fucosylation. Several reports reveal that fucosylation plays an important role in many diseases such as pancreatic cancer, LCH and HCC [123, 124]. Another recent study examined the galectin-1 binding ability of Hp in the sera of metastatic breast cancer patients, where the binding was twice as strong, possibly due to a difference in glycoforms [125, 126].
It is interesting to see that two studies from the same year report different glycosylation patterns for Hp [119, 120]. This might be caused by a different ethnicity of the sample donors, as one study has been performed in China and the other in the United States, two geographical regions that have been reported to have different phenotype distributions [118]. That difference has not yet been taken into account in glycomics studies.
Hemopexin (P02790)
Hemopexin (HPX), also known as beta-1B-glycoprotein, is a 462 amino acid (23 are part of the signal peptide) single polypeptide chain plasma glycoprotein with a peptide backbone of 51 kDa and an apparent mass ranging from 57 to 80 kDa depending on its glycosylation [315–317]. The protein is mainly expressed by the liver and found in serum at levels of 0.8 mg/mL in adults, while levels in newborns have been measured around 20 % of that value [318, 319]. It is also expressed in the central nervous system, in the retina and in the peripheral nerves. The protein structure is controlled by six disulfide bridges next to its glycosylation [316]. HPX is an acute phase response glycoprotein, capable of binding heme with the highest known affinity of all plasma proteins. When a heme is captured, the complex can be recovered from plasma by the HPX receptor (such as found on the membrane of liver parenchymal cells) leading to internalization, catabolization of the heme and recycling of the proteins involved. After the process, HPX is free to return to the circulation. HPX is found to be expressed in large quantities in case of inflammation, a state in which heme is highly abundant in plasma. As heme would otherwise induce oxidative stress, the function of HPX can be described as antioxidant [316].
Glycosylation
HPX contains five confirmed N-glycosylation sites located at Asn64, Asn187, Asn240, Asn246 and Asn453 [59, 60, 70, 127–130]. In general, plasma HPX N-glycosylation consists mainly of diantennary structures with high levels of galactosylation, while low levels of triantennary and fucosylated structures have also been reported [67, 121, 131–133]. The degree of sialylation of the protein remains to be fully investigated, but lectin capturing of α2-6-sialylated HPX glycopeptides followed by LC-MS(/MS) analysis has revealed the presence of fully sialylated antennae and only low levels of monosialylated diantennary glycans [68]. Combining the information, the main glycan composition on HPX is expected to be A2G2S2. Site-specific characterization has been achieved on a compositional level for N-glycosylation sites Asn64, Asn187 and Asn453, each of them showing similar ratios of glycoforms (85–94 % diantennary nonfucosylated, 4–7 % diantennary fucosylated, as well as low levels of triantennary structures) [62, 121]. Asn240 and Asn246 remain uncharacterized, likely due to their close proximity. The antennarity and the degree of fucosylation (core and antennary) have been reported to increase with LCH and HCC [134]. Notably, HPX also contains two O-glycosylation sites (Thr24 and Thr29) one of which is located on the N-terminal threonine (after removal of the signal peptide), and a potential minor O-glycosylation in the Ser30-Thr40 region [62, 127, 130].
Histidine-rich glycoprotein (P04196)
Histidine-rich glycoprotein (HRG), also called histidine-proline-rich glycoprotein (HPRG), has an apparent molecular mass of 72 kDa (peptide backbone of 60 kDa) and consists of 525 amino acid (507 without the signal peptide) [320, 321]. The protein occurs in plasma at concentrations of 0.1–0.15 mg/mL, and is mainly produced by the liver parenchymal cells although some reports suggest synthesis in immune cells as well [322–325]. Levels in newborns are only approximately 20 % of those in adults [326]. HRG is known to regulate immunity, coagulation and angiogenesis [327]. To achieve this, it interacts with many different ligands including heme, heparin, plasminogen, fibrinogen, thrombospondin and immunoglobulin G, as well as many cell surface receptors and divalent cations such as Zn2+ [325]. It is a negative acute phase protein, showing decreased plasma levels during inflammation, injury or pregnancy [328].
Glycosylation
HRG is expected to have a large degree of glycosylation, as 14 % of the protein weight (around 10 kDa) has been attributed to the oligosaccharide portion [135]. Three N-glycosylation sites have been confirmed by glycoproteomic analysis, located at Asn63, Asn125 and Asn344 [60, 70]. Another glycosylation site is theoretically present at Asn345, but the direct vicinity with the site Asn344 may sterically hinder its occupation and additionally complicates its analysis. Interestingly, a common polymorphism can induce a new glycosylation site at Asn202 by replacing a proline by a serine at position 204, creating the motif Asn-X-Ser, and N-glycanase treatment revealed a mass difference of 2 kDa attributed to the new Asn202 carbohydrate compared to the unmodified form of HRG [136]. The sequence Asn87-Asp-Cys found in HRG has been reported to contain glycosylation (in bovine protein C) but no clear evidence of its presence has yet been made for human HRG [137, 138]. With regard to the classical sites, little is known, and to the best of our knowledge, neither site occupancy nor relative abundance of glycan structures have been studied. However, if a carbohydrate mass of 10 kDa needs to be distributed across three glycosylation sites, the average site would contain glycans of over 3300 Da (putting them into the tri- and tetraantennary range with high levels of galactosylation, sialylation and/or fucosylation). No reports about changes in glycosylation under disease conditions were found for HRG.
Kininogen-1 (P01042)
Kininogen-1, also called alpha-2-thiol proteinase inhibitor, Fitzgerald factor, high-molecular-weight kininogen (HMWK) or Williams-Fitzgerald-Flaujeac factor, has a single polypeptide chain of 644 amino acid (18 belonging to the signal peptide) and approximates 114 kDa apparent molecular mass (while its theoretical mass without glycosylation is 70 kDa) [139]. Kininogen can be cleaved into six different subchains called kininogen-1 heavy chain, T-kinin (Ile-Ser-bradykinin), bradykinin (kallidin I), lysyl-bradykinin (kallidin II), kininogen-1 light chain, and low molecular weight growth-promoting factor. In its intact form, the protein is a cysteine proteinase inhibitor, implicated in blood coagulation and inflammatory response and it can bind calcium, while the individual subchains can have many other functions [329–332]. Kininogen is mainly synthetized in the liver to plasma concentrations of 55–100 μg/mL, where it is mostly found in complex with prekallikrein or factor XI to position the coagulation factors near factor XII [331, 333–336].
Glycosylation
Kininogen has four N-linked glycosylation sites at Asn48, Asn169, Asn205, Asn294 (all of which remain on the kininogen-1 heavy chain after cleavage) and eight O-linked glycans at sites Thr401, Thr533, Thr542, Thr546, Thr557, Thr571, Ser577 and Thr628 [59, 60, 62, 70, 139]. While no overall or site-specific glycosylation analysis has been performed yet, core-fucosylation has been reported for sites Asn48, Asn205 and Asn294 on the basis of RP LC-MSn after capturing with L. culinaris lectin [67]. In addition, two-dimensional gel electrophoresis, with staining for triantennary structures carrying sialyl-Lewis X has demonstrated the natural presence of this epitope on the protein, as well as its upregulation in patients with stomach cancer [140]. Kininogen is expected to be highly glycosylated by large N- and O-glycan structures, as the observed protein mass is more than 40 kDa higher than the mass calculated from the amino acid sequence. Kininogen glycosylation changes due to diseases have not yet been described.
Serotransferrin (P02787)
Serotransferrin (STF), also known as transferrin, β1 metal binding globulin or siderophilin, is a 698 amino acid protein (19 amino acids of which are signal peptide) with a molecular mass of approximately 77 kDa (without glycosylation) [8, 337]. The protein consists of two globular domains, the N-lobe and the C-lobe which divided into two subdomains each (N1, N2, C1 and C2). The two main domains are connected by a short linker peptide [337–339]. The N-lobe is 336 amino acids in size and spans from Val25 to Glu347, while the C-lobe is 343 amino acids long and ranges from Val361 to Lys683 [337]. The lobes can interact to form a hydrophilic metal ion binding site [337]. STF is mostly produced by hepatocytes, although other tissues have also shown expression, albeit at significantly lower amounts [337]. The plasma concentration is highly stable from the age of 2 years on, with a range between 2 and 3 mg/mL [337, 340]. Levels may increase during pregnancy up to 5 mg/mL [141].
STF is an iron binding protein and it regulates iron levels in biological fluids. It can bind two Fe3+ ions and transport those throughout the body, avoiding the toxicity of free radical formation that may be caused by free Fe3+ ions [337]. Iron is essential for DNA replication as it is a co-factor of ribonucleotide reductase [341]. Several studies have shown that the number of transferrin receptors at the surface of cells was closely correlated with their proliferation state and their iron status [142, 342]. In addition, STF has been associated with several diseases like atransferrinemia and cardiovascular diseases [337]. In inflammation and allergic reactions, the STF levels are found to be significantly reduced in plasma [337]. The protein has also shown potential as a therapeutic agent. For instance, oxidative damage caused by radiotherapy can be reduced by infusion with apo-transferrin [343]. The proprieties of STF and its receptor can be exploited to deliver drugs specifically into the brain and cancer cells [344]. Additionally, conjugates consisting of the protein and a drug have been shown to yield high specific cytotoxicity (e.g. Tf-ADR versus HeLa, HL-60 and H-MESO-1 cell lines) [344, 345].
Glycosylation
STF has two N-glycosylation sites located at Asn432 and Asn630 and a potential minor site at Asn491 (Asn-X-Cys) [8, 59, 141, 143, 144]. Around 6 % of the total weight of the protein is due to the carbohydrate content [142]. Lectin mobility (ConA – Sepharose column) followed by sequential exoglycosidase treatments on two STF samples of healthy patients showed that, overall, the main glycans are A2G2S2 (96–97 %), FA2G2S2 (2–3 %) and A3G3S2 (1 %) [145]. The glycosylation per site has been studied using nano-LC-ESI-MS combined with exoglycosidase treatment [143, 144]. The sites at Asn432 and Asn630 proved to be the main contributors to the total glycome, while the non-standard glycosylation site at Asn491 was glycosylated at a level of approximately 2 % [143, 144].
The glycans present on Asn432 are A2G2S2 (93.5 %), A3G3S2 (2.5 %), A2G2S1 (2.4 %) and A2FG2S2 (1.6 %), while Asn630 contains A2G2S2 (85.9 %), FA2G2S2 (6.9 %), A2FG2S2 (2.8 %), A2G2S1 (2.2 %), A3G3S2 (1.0 %), as well as some lower abundant species with increased fucosylation [143, 144]. The fucosylated antenna is most likely of sialyl-Lewis X type (at the α1-3-linked arm, β1-4-linked antenna) as it was shown by NMR spectroscopy of material purified from the amniotic fluid of pregnant women [146]. A single type of glycosylation was detected on the minor glycosylation site at Asn491, namely A2G2S2 [143]. STF N-glycosylation has been investigated in other biologic fluids like CSF, where similar structures have been found along with some disease-related ones [8, 147].
The glycosylation of STF has shown to be different across fluids and phenotypes with the abundance of A2G2S2 being significantly reduced in human amniotic fluid (55 %) or in the plasma of hepatoma patients (37–63 %) [145, 146]. The percentage of triantennary structures is largely increased in amniotic fluid to 32 %, while the abundance of triantennary structures in the serum of hepatoma patients ranges from 21 to 63 % [145, 146]. Abnormal isoforms of serotransferrin, especially variation in the sialic acid content, are a very sensitive and reliable biomarkers of many CDGs, and potentially for idiopathic normal pressure hydrocephalus (iNPH) patients [147, 148]. Isoelectric focusing (IEF) of serotransferrin is the first test used to rapidly reveal N-glycosylation related CDGs while apolipoprotein C-III is the protein of choice for the test of O-glycosylation related CDGs [149, 150]. Interestingly, carbohydrate deficient STF levels can also be used as indicator of heavy alcohol usage, even post mortem [8, 151].
Vitronectin (P04004)
Vitronectin (VN), also called S-protein, serum spreading factor, or V75, is a 459 amino acid 52.4 kDa member of the pexin family and of the adhesive glycoproteins group [346–349]. The apparent molecular mass of 75 kDa is due to post translational modifications including glycosylation. VN is mainly produced in the liver and it is found in plasma at concentration of 0.2–0.4 mg/mL, where it is mostly present in monomeric or dimeric form [34, 348]. VN is also found in other body fluids such as seminal plasma, urine, amniotic fluid, CSF, bronchoalveolar lavage fluid and in platelets [348].
VN is an adhesive glycoprotein, and shows a role in blood coagulation, extracellular matrix binding, regulation of cell adhesion and spreading, and innate immunity [346, 347]. It also protects the membrane from the damages caused by the terminal cytolytic complement pathway. Underexpression of the protein has been correlated with liver conditions like fibrosis, while elevated levels have been reported in inflammatory states [350–352]. VN is also found to be implicated in HCC where specific glycoforms have been identified [152].
Glycosylation
Three N-glycosylation sites have been identified in VN at Asn86, Asn169 and Asn242 by LC-MS(/MS) [59, 70]. Without site specificity, the major VN carbohydrate forms reported by LC-fluorescence are diantennary and triantennary complex type glycans, with a low percentage of hybrid structures [153]. Sialic acids are mainly found α2-6-linked, as determined by sialidase and acid treatments, followed by NMR. About 19 % α2-3-linkage has been detected on the α1-6-arm of the diantennary structures and on the β1-6-linked N-acetyllactosamine of the α1-3-arm of triantennary structures. Core fucosylation of vitronectin accounts for 7.9 % [153].
When looking at the glycosylation in a site-specific manner by trypsin digestion and LC-ESI-MS(/MS) analysis, Asn86 shows mainly A2G2S2 species (45 %), as well as A3G3S3 (33 %) and A3FG3S3 (20 %) [152]. At Asn169, a higher variety of glycan structures are observed. Next to the fully sialylated diantennary structures (A2G2S2, 76 %) and its monosialylated variant (6 %), around 18 % sialylated hybrid structures have been detected (ranging from 3 to 5 mannoses). Asn242 bears diantennary di- and monosialylated N-glycans (A2G2S2, 50 %; A2G2S1, 20 %), with possible core fucose on the fully sialylated variant (FA2G2S2, 10 %). In addition, triantennary fully sialylated structures have been detected with and without fucose (A3G3S3, 10 %; FA3G3S3, 10 %) [152].
Core fucosylation of VN has been reported at Asn242 in healthy individuals and on Asn86 in HCC patients [67]. Hybrid type and fucosylated glycans of VN have been reported to increase in patients suffering from HCC and other cancers, and thus shows potential as biomarker [152, 154]. A possible explanation for the increase of hybrid type glycans is that the alpha mannosidase in the Golgi apparatus is suppressed in HCC [17].
Zinc-alpha-2-glycoprotein (P25311)
Zinc-alpha-2-glycoprotein (ZAG, not to be confused with ZAG which is the short name of its AZGP1 gene), also abbreviated Zn-alpha-2-glycoprotein or Zn-alpha-2-GP, is a 41 kDa glycoprotein (15 % of the mass being carbohydrate) comprising a single 298 amino acid chain (20 amino acid signal peptide), with two intra-chain disulfide bridges [155, 353, 354]. The protein is produced by the liver and occurs in plasma at concentrations around 0.03–0.11 mg/mL with a mean at 0.05 mg/mL. As with many plasma glycoproteins, the functions of ZAG are diverse. The protein has been shown to interact with the beta-3-adrenoreceptor on adipocyte cells, inducing the depletion of fatty acids [355]. While its serum variant originates from hepatocytes, ZAG is expressed in many cell types including adipose tissue, buccal cells and prostate epithelial cells, and occurs in many body fluids like seminal fluid where its concentration is six time higher than in serum [355]. Functions of the on-site produced ZAG include fertilization, melanin production, regulation of the immune response, and many others. In addition, the serum concentration of ZAG shows a large increase in various types of cancer, making it a particularly good biomarker for female breast and male prostatic carcinomas [355].
Glycosylation
Four N-glycosylation sites have been detected on ZAG at Asn109, Asn112, Asn128 and Asn259 [59, 60, 70, 129, 156, 157]. For three of the sites (Asn112, Asn128 and Asn259) proton nuclear magnetic resonance (1H-NMR) spectroscopy has revealed the major N-glycan structure to be diantennary and disialylated A2(2)G2(4)S2(6) [155]. For Asn259 specifically, partial sialylation (90 %) of the α1-6-linked antenna has been reported. The general presence of diantennary N-glycans, and the sialylation thereof, has been verified by proteomic experiments, but to date no extensive study has been made on its glycan microheterogeneity [96, 158]. Asn109 and Asn128 have for instance been suggested to carry in part core fucosylated N-glycans, but this has hitherto remained unconfirmed [67]. No information about the effect of diseases on ZAG glycosylation was found in literature.
Immunoglobulins
Immunoglobulins (Igs) are a major component of the adaptive immune system [159]. There are five distinct classes in humans (IgA, IgD, IgE, IgG and IgM), which all share common components. Generally, immunoglobulins consist of two heavy chains and two light chains. These chains contain one variable part (HL or VL, respectively) and three or more constant domains on the heavy chain (CH n), or one on the light chain (CL). Furthermore, immunoglobulins can be subdivided into a fragment antigen-binding (Fab) and a fragment crystallizable (Fc) portion. The Fab domain consists of the VH and VL domains and the adjacent N-terminal constant CH1/CL domain. The Fc domain is built up of the remainder of the heavy chains. Immunoglobulins thus contain two Fab domains per Fc domain. Each immunoglobulin has its own specific heavy chains (α, δ, ε, γ or μ) which are joined by one or more disulfide bridges. The light chain can occur in two variants (λ and κ) that are shared by all immunoglobulins. Some immunoglobulins additionally contain a flexible hinge region between the CH1 and CH2 domains (IgA, IgD and IgG). The remaining immunoglobulins (IgE and IgM) have a rigid Ig domain instead of a hinge region. Immunoglobulin N-linked glycosylation occurs mostly on the heavy chains, accounting for between 2 and 14 % of the protein weight. However, the light chain can also contain N- and O-linked glycans [159].
Immunoglobulin A (P01876; P01877)
Immunoglobulin alpha (IgA) is an antibody that exists in two subclasses (IgA1 and IgA2), and in both mono- and dimeric form. Compared to IgA2, IgA1 contains a 13 amino acid extended hinge region, which is heavily O-glycosylated [356, 357]. Serum IgA consists mostly of the 160 kDa IgA monomer (mIgA), has a concentration of 2.62 mg/mL (of which approximately 90 % is IgA1), and is produced by the bone marrow [357, 358]. Secretory IgA (sIgA) is observed at mucosal surfaces and produced locally, mainly occurring as a dimer of two mIgA units and a set of two connecting peptides, the J-chain and the secretory component [356, 358]. Secretory IgA is a key player in the immune defense at mucosal surfaces. Pathogenic microorganisms are prevented from attaching to the mucosal surface by sIgA surrounding the pathogen, which is then repelled by the mucosal surface due to the high abundance of hydrophilic amino acids and glycosylation [356]. The precise role of IgA in the circulation is not clear.
Glycosylation
IgA is N-glycosylated at Asn144 (IgA1) and Asn131 (IgA2) in the CH2 domain as well as at Asn340 (IgA1) and Asn327 (IgA2) and in the tail piece domain [160]. IgA2 contains two additional N-glycosylation sites at Asn47 of the CH1 domain and at Asn205 of the CH2 domain [160]. While glycosylation studies have been performed for IgA1, the glycosylation of IgA2 remains to be characterized.
The main N-glycans present on IgA1 as detected by hydrophilic interaction liquid chromatography (HILIC)-HPLC with exoglycosidase digestion are diantennary disialylated (A2G2S2, 24 %), diantennary monosialylated (A2G2S1, 20 %) and fucosylated diantennary bisected disialylated (FA2BG2S2, 14 %) [161]. The amount of nonsialylated glycans detected was marginal, being at levels for total IgA of 6 % for the Fc region and 2 % for the Fab region [161]. A site-specific study showed that the fucosylated glycans are mostly present on the Asn340 site [162]. Furthermore it was shown that the main glycoform on the IgA1 Asn144 containing glycopeptide was A2G2S1, while the Asn340 containing glycopeptide carried mainly the FA2G2S2 [163].
The role of N-glycosylation of IgA in diseases is not well understood. The glycan might have an influence on the binding of IgA to the FcαR receptor, although this finding was not validated in a later study [161, 164]. Binding to the FcαR receptor can induce a pro- or anti-inflammatory response [165]. In addition, the presence or absence of sialic acids on the glycans may influence the clearance of IgA from the circulation by the asialoglycoprotein receptor [159].
Immunoglobulin D (P01880)
Immunoglobulin delta (IgD) has, compared to the other immunoglobulins, a rather long hinge region of 64 amino acids resulting in a total apparent mass of 175 kDa. The average concentration of IgD in plasma is 0.03 mg/mL, but it can range from <0.003 to 0.4 mg/mL without any clear sex or age dependence [359–361]. Compared to the other immunoglobulins, it has a short half-life of 2.8 days [362]. IgD can be found in a secreted isoform (sIgD), as well as membrane bound on immature B cells [363]. The protein is involved in immunity and inflammation, by binding to respiratory bacteria, resulting in clearance [32].
Glycosylation
Three N-glycosylation sites have been identified on the heavy chain of IgD, located at Asn225, Asn316 and Asn367, as well as seven O-glycosylation sites at Ser109, Ser110, Thr113, Thr126, Thr127, Thr131 and Thr132 [166–168]. HILIC-HPLC analysis with exoglycosidase digestion on released IgD N-glycans revealed a mixture of high mannose and complex type glycosylation. The total pool contained 35 % core fucosylation, <1 % terminal N-acetylglucosamine, 33 % bisection, 20 % terminal galactosylation and 31.5 % monosialylation and 21.5 % disialylation (sialic acids being α2-6-linked). The most abundant glycoforms detected were Man8 (14.4 %), Man9 (13.5 %), FA2G2S2 (7.6 %), FA2G2S1 (7.3 %), FA2BG2S2 (6.5 %) and A2G2S1 (6.1 %). Interestingly, monoglucosylated Man8 and Man9 (Man8Glc, Man9Glc; 2.4 %, 3.3 %) were observed as well [169]. The high mannose glycans were preferably found at the Asn225 site, while the complex type glycans were abundant at Asn316 and Asn367 [168]. The absence of glycans at Asn225 has been shown to completely inhibit the secretion of IgD [170].
Immunoglobulin E (P01854)
Immunoglobulin epsilon (IgE) is a 188 kDa antibody lacking a hinge region, but that instead contains an extra C domain in its heavy chain [364]. The protein exists as a membrane-bound receptor form and in a soluble form [365]. IgE has a serum concentration of around 0.3 μg/mL, making it the lowest abundant immunoglobulin [366]. No in-depth study has yet been performed to elucidate where IgE is synthesized [364]. The primary function of IgE is the induction of an anti-parasitic immune response by activation of mast cells and basophils through the FcɛRI receptor [367]. This mechanism is also proposed to play a role in the formation of allergic responses [366, 368].
Glycosylation
Carbohydrates form 12 % of the IgE molecular mass, making it the most glycosylated antibody in plasma [171]. The protein contains six N-glycosylation sites, located at Asn21, Asn49, Asn99, Asn146, Asn252 and Asn275 [172]. An additional potential site at Asn264 was not found to be glycosylated [173]. HILIC-HPLC with exoglycosidase digestion of 2-aminobenzamide-labeled glycans showed the overall species to be mainly core-fucosylated diantennary with either one sialic acid (FA2G2S1) or two (FA2G2S2) (18 and 25 % respectively) [169]. These glycans have, to lesser extent, been found in non-fucosylated form (A2G2S1, 11 %; A2G2S2, 11 %), and with bisecting N-acetylglucosamine (FA2BG2S1, 8.2 %). In addition, 14.2 % of the glycan pool proved to be high-mannose type (mainly Man5) [169].
By performing site-specific analysis by LC-MS/MS of tryptic glycopeptides, it was found that Asn21 mainly contains FA2G2S1 (30 %), FA2BG2S1 (30 %), FA2G2S2 (15 %) and FA2BG2S2 (10 %), while Asn49 is occupied by FA2G2S2 (30 %), FA2G2S1 (18 %), FA2BG2S2 (15 %), and FA2BG2S1 (15 %) [173]. Asn99 contains FA2G2S2 (40 %), FA2G2S1 (20 %) and bisected species in lower relative abundance (<10 %). Asn146 is glycosylated by FA2G2S2 (50 %), FA2BG2S2 (30 %), and around 10 % of FA2G2S1, while Asn252 is highly bisected, mainly containing FA2BG2S1 (35 %) and FA2BG2S2 (25 %), as well as a lesser amount of the nonbisected species FA2G2S2 (15 %) and FA2G2S1 (10 %) [173]. Interestingly, the Asn275 site almost exclusively shows oligomannosidic structures, the main species being Man5 (50 %), but higher numbers of mannoses are found as well (Man6, 15 %; Man7, 10 %; Man8, 10 %; Man9, 5 %) [171, 173].
In the same study, IgE from hyperimmune donors was seen to be similarly glycosylated as normal IgE, while IgE derived from monoclonal myelomas showed the loss of bisection and a drastic appearance of triantennary structures (up to 50 % per site) [173]. There is evidence that IgE glycosylation is important in binding to the FcϵRI receptor and can be implicated in the initiation of anaphylaxis [174, 175]. In contrast, other sources indicate that the glycans in the Fc region are only minor contributors to the binding of IgE to the FcϵRI receptor [176].
Immunoglobulin G (P01857; P01859; P01860; P01861)
Immunoglobulin gamma (IgG) is a glycoprotein with a total molecular mass of approximately 150 kDa [159, 177]. The light chain consists of a domain covering the variable region (VL) as well as a constant (CL) domain. The heavy chain contains four domains with one domain which comprises the variable region (HL) followed by three constant domains: CH1, CH2 and CH3. Each light chain is paired with the HL and CH1 domain of the heavy chain to form a Fab portion, whilst CH2 and CH3 domains of the two heavy chains together form the Fc portion. Between the two Fab portions and the Fc portion a flexible hinge region is positioned, which makes it possible for the two Fab arms to move individually [159, 364]. The antibody is highly stable with a half-life of approximately 12 days [369, 370]. Based on the amino acid sequence of the constant regions of the heavy chains, IgGs can be divided into four subclasses namely, IgG1 (P01857), IgG2 (P01859), IgG3 (P01860) and IgG4 (P01861) [371, 372]. Notably, IgG3 has a larger hinge region (62 amino acids) compared to the other subclasses (12 amino acids).
During a secondary immune response IgG is secreted in high amounts by B cells [373]. In healthy individuals the concentration of IgG in serum is between 7 and 18 mg/mL [374]. The average subclass-specific concentrations in plasma are reported as 5.03 mg/mL for IgG1, 3.42 mg/mL for IgG2, 0.58 mg/mL for IgG3 and 0.38 mg/mL for IgG4 [375]. IgG molecules are important for activating the complement system through the classical pathway (antibody-triggered) as well as binding to specific receptors on macrophages and neutrophils [364, 373]. The IgG subclasses differ in their ability to activate the complement system. The primary activators of the complement system are IgG1 and IgG3, whereas IgG2 can also activate it at a lower level. IgG4, on the other hand, is not capable of activating the complement system [364]. Furthermore, IgG molecules are the only antibodies that can pass from a mother to her child via the placenta, and maternal IgG has been shown to gradually decrease throughout pregnancy [364, 376, 377].
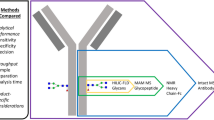
The majority of therapeutic antibodies is derived from IgG1, where the glycosylation plays an important role for their function [35, 364, 377–380].
Glycosylation
Glycosylation can occur on both the Fc and Fab portions of the IgG molecules [178]. The Fc region has been extensively studied with a highly conserved N-glycosylation site in the CH2 domain at Asn297. Notably, this site may have a different number for different IgG subclasses and variants [178].
Another possible N-glycosylation site may be found at Asn322 of IgG3 although no occupation has yet been described [178]. The Fab portion is known to be N-glycosylated in 15–25 % of the cases [179].
The overall glycosylation of IgG has been the subject of many studies using a variety of different methods [24]. In a recent glycosylation MALDI-TOF-MS study on a released glycan level, the most abundant glycans were complex types, i.e. FA2G1 (31 %), FA2G2 (23 %), FA2G2S1(6) (13 %), FA2 (10 %) and FA2BG1 (5 %) [180]. Only a small portion of the glycans were found to be high mannose (0.21 %), of which Man8 (0.06 %) was the most abundant, followed by Man9 (0.05 %). Overall, 92 % of the total IgG pool was core-fucosylated, 13 % bisected, 18 % monosialylated and 3 % disialylated. Twelve percent of the glycans contained α2-6-linked sialic acids, against 0.2 % α2-3-sialylated species [180]. These findings are in agreement with previously reported sialylation values obtained by lectin interaction [181]. Next to the study of overall IgG glycosylation, differences between the Fab and Fc have also been studied by MALDI-TOF-MS after affinity capturing of the different regions [180]. The Fc region shows a similar profile as the total IgG profile, albeit with a lesser degree of sialylation. FA2G1 (32 %) was again the most pronounced glycan, followed by FA2G2 (27 %), FA2G2S1(6) (15 %), FA2 (9 %) and FA2BG1 (5 %), while the amount of high mannose type species was found to be very low (0.1 %). In contrast to Fc, the Fab region showed a significantly higher degree of sialylation, with 40 % of the species being monosialylated, and 52 % being disialylated. Also bisection and high mannose species were seen to be higher (45 and 4 % respectively). Specific compositions included FA2BG2S1(6) as most abundant with 21 %, followed by A2G2S2(6) (17 %), FA2BG2S2(6) (16 %), FA2G2S2(6) (16 %) and FA2G2S1(6) (10 %). For the high mannose types Man6 was the most abundant with 1.2 % followed by Man8 (1.0 %) and M5 (0.7 %) [180].
Affinity capturing followed by LC-MS with CID and electron-transfer dissociation (ETD) fragmentation of tryptic IgG glycopeptides revealed that the various IgG subclasses are similarly glycosylated, but with some notable differences [182, 183]. IgG1 tends to show higher galactosylation than the other subclasses, whereas IgG2 shows the highest degree of core-fucosylation and IgG3 the least. IgG4 was found more difficult to study due to its relatively low abundance [182]. Another study examined the O-glycosylation of IgG3 in the hinge region, revealing that the threonine sites (T) in the three repeated peptide sequences (CPRCPEPKSCDTPPP) are partially occupied with core 1-type O-glycans [184].
A vast body of literature exists describing disease-associated changes of IgG glycosylation, as well as the regulation and immunological effects of such glycosylation changes. In the following, only a very concise view of this field will be given, and we would like to refer the interested reader to more specialized reviews [177, 185, 186].
IgG glycosylation has been strongly associated with age, with a negative correlation between age and galactosylation [19, 20, 187, 188]. IgG FA2 seems to have a strong pro-inflammatory effect through various mechanisms, e.g. the lectin pathway of the complement system [19, 188, 189]. Increased levels of FA2 glycans and/or lowered levels of FA2G2 glycans is found in many diseases, including rheumatoid arthritis, Crohn’s disease, granulomatosis with polyangiitis, tuberculosis, HIV and myositis [189–193]. Several studies revealed that core-fucosylation is an important factor in the binding capacity of the Fc region to the FcϒRIIIa receptor [194, 195]. The lack of core-fucosylation suggests improvement in the binding extensively resulting in a higher degree of antibody-dependent cell mediated cytotoxicity (ADCC) receptor [194, 195]. The presence of sialic acids is able to reduce the binding capacity of the antibody to the FcϒRIIIa receptor, as a consequence the activity of ADCC is decreased and anti-inflammatory effects are enhanced, although this only appears to be the case for α2-6-linked sialylation [33, 178, 196, 197]. Interestingly, during pregnancy the glycosylation also appears to change, especially in the Fc region where the levels of galactosylation and sialylation increase [28, 198–200] . This might be to suppress the immune response of the mother against her child [198]. Alterations in glycosylation have also been reported to occur in a subclass specific level, for example in patient suffering from hepatocellular carcinoma, cirrhosis, or myositis [190].
Immunoglobulin M (P01871)
Immunoglobulin mu (IgM) is a 970 kDa (in pentameric form) antibody, consisting of five 190 kDa subunits (of 452 amino acids) [159, 364, 381]. The protein can be membrane-bound on the B1- and B2-cells where they are produced [382], or secreted into blood at plasma concentrations of 0.5–2.5 mg/mL [374]. In circulation, IgM exists either as a pentamer with coupling J-chain, or occasionally as hexamer lacking the J-chain [383]. No hinge region has been reported for the immunoglobulin, but it contains an extra C domain instead [364]. In total, pentameric IgM consists of 10 heavy chains, 10 light chains and 1 J-chain, arranged in a mushroom-shaped molecule, leading up to being the largest antibody in human plasma by far [384].
IgM antibodies are an early and main activator of the classical complement pathway [364], but also play a role in homeostasis, inflammation, infection, atherosclerosis and autoimmunity [381]. The protein is additionally involved in apoptosis [381], as absence of IgM shows a three- to four-fold decrease in apoptotic cell uptake by macrophages [385]. Furthermore, IgM has been described in several studies regarding acute coronary syndromes and cardiovascular diseases, where an elevated urine excretion of IgM has been reported [386, 387]. The presence of glycans on non-antigen bound IgM may also assist the agglutination of virus particles present in serum via viral lectin hemagglutinins [159].
Glycosylation
IgM contains N-glycosylation sites at Asn46, Asn209, Asn272, Asn279 and Asn439, of which Asn439 is only 17 % occupied [159, 201, 202]. HILIC-HPLC-MS has shown the overall N-glycosylation to be mainly diantennary with core fucosylation, either with- or without bisecting N-acetylglucosamine (FA2BG2S1, 26 %; FA2G2S1, 19 %), followed by the oligomannose compositions Man6 and Man5 (10 and 6 %, respectively) [202]. By lectin capturing, the high-mannose compositions have been attributed to Asn297 and Asn439, leaving the complex type glycosylation to be found at Asn209 and Asn272 [159, 203]. Interestingly, the partially occupied Asn439 shows larger high-mannose structures (Man6-8) than site Asn297 (Man5-6), suggesting that the former is difficult to access by both the dolichol precursor and the mannosidases required for trimming of the structure. Depending on the source, Asn46 can be occupied by either oligomannose or complex type N-glycans [159, 203].
Discussion
Here we present an overview of the N-glycosylation of 24 major plasma glycoproteins. It has been shown for many of these proteins that glycosylation changes are implicated in serious pathological states such as cancers, autoimmune diseases and CDG and that their glycosylation pattern could be used as biomarkers, prognostic tools or even as anchor points for targeted treatments [15, 35, 148, 388, 389]. These findings have resulted in an increasing interest in the glycosylation analysis of easily accessible biofluids such as plasma, and many correlations have been established between disease (states) and the abundance of specific glycosylation traits. Recent advances in sample preparation methods, separation techniques, mass spectrometry, and the development of robotized platforms are easing routine analysis of the total plasma N-glycome and allow the screening of large cohorts in reasonable times, thus enhancing the possibilities to search for putative biomarkers and predictions tools [38, 109, 390–392]. This, however, requires that the observed differences in glycosylation can be interpreted in a biologically meaningful manner, and traced back to changes in the levels of glycosylated proteins, and to possible glycosylation changes of specific proteins.
The information contained in this review stems from many sources and methodologies. When exploring protein glycosylation to its full complexity, this comprises the occupancy per site and the relative abundances of glycoforms present per site, as well as information on linkages and isomer distribution. No single analysis method is capable of providing all this information in a comprehensive manner, let alone on a complex sample such as human plasma. Consequently, different levels of detail are available with respect to the glycosylation of the major glycoproteins that make up human plasma. Analysis methods encountered in this review are very diverse, including NMR, lectin capturing, LC-fluorescence, as well as several mass spectrometric methods [72, 390, 393]. NMR has proven definitive for providing structural features of a glycan, but is limited by sample throughput and amount of material needed, as well as by its inability to characterize multiple species present in a complex sample [390]. Mass spectrometric methods applied in bottom-up studies of glycopeptides generally only provide glycosylation information on a compositional level, but may in addition provide site-specific glycosylation information by revealing the amino acid sequence as well as glycan attachment site [182]. As such, high-throughput mass spectrometric screening methods have been used to identify and confirm many of the glycosylation sites in human plasma, although the use of deglycosylated peptides has prevented characterization of the glycans themselves [59, 60, 67]. A particularly successful combination of methodologies for in-depth study of glycosylation has proven to be LC-MS(/MS) with exoglycosidase digestion and/or lectin capturing, which has been used to study a fair number of the proteins covered in this review [110, 169].
Well-studied proteins covered in this review include alpha-1-acid glycoprotein, alpha-1-antitrypsin, haptoglobin, serotransferrin, vitronectin, and IgG, while others such as histidine-rich glycoprotein and kininogen-1 still remain to be studied at the most basic level (Table 1). Overall characterization reveals a high degree of galactosylation and sialylation across the plasma N-glycome, the most abundant species for most sites having full coverage of all their antennae. Next to receptor interaction and charge induction, the terminal sialic acids are known to play a large role in determining the half-life of proteins, and less than full sialylation would lead to hepatic clearance via the asialoglycoprotein receptor [394].
Specifically, the major detected glycan species are A2G2S2 and its monosialylated variant (Fig. 1, Table 1), with β1-2-linked antennary N-acetylglucosamines, β1-4-linked galactoses, and α2-6-linked N-acetylneuraminic acids [104, 155]. These are found on the majority of glycoproteins, and are particularly abundant on serotransferrin, fibrinogen, ceruloplasmin and alpha-2-macroglobulin. Potential fucosylation for these diantennary structures mostly occurs in α1-6-linkage on the core N-acetylglucosamine.
The more truncated N-glycosylation, i.e. lack of sialic acid termini and incomplete galactosylation, is reserved for immunoglobulin G, and to lesser extent apolipoprotein B-100 [11, 180]. Interestingly, immunoglobulin G is the only major plasma protein we have found to contain core-fucosylation as well as incomplete galactosylation/sialylation, meaning that the TPNG species FA2, FA2G1 and FA2G2 predominantly reflect the glycosylation of this protein [180]. Similarly, immunoglobulin M is the major carrier of the bisected species FA2BG2S1, with IgG and the lowly abundant immunoglobulin E contributing to the expression of this compositional glycan to a lesser extent [169, 180, 202].
The high mannose type glycosylation is also differentially distributed. Whereas the lower size oligomannose structures Man5 and Man6 are distributed across alpha-2-macroglobulin, apolipoprotein B-100 and immunoglobulin M, the larger structure Man9 mainly originating from apolipoprotein B-100 [11, 72, 202]. In addition, the high mannose structures have been reported on the Fab portion of IgG [180].
Tri- and tetraantennary structures are found in lower abundance than the diantennary structures, and have some discerning features. Whereas diantennary glycans are mainly sialylated with an α2-6-linkage, for the triantennary species on average one in three sialic acids is α2-3-linked [54]. Furthermore, potential fucosylation is predominantly α1-3-antennary, and located at the α2-3-sialylated antenna to form sialyl Lewis X, which itself is favored on the β1-4-linked N-acetylglucosamine of the α1-3-branch [54]. These fucosylated and non-fucosylated triantennary glycans are commonly expressed in minor amounts for sites that also have diantennary glycosylation (examples including alpha-1-antitrypsin and ceruloplasmin), but represent the most abundant glycosylation type for alpha-1-acid glycoprotein. Among the proteins covered in this review alpha-1-acid glycoprotein also stands out by expressing tetraantennary N-glycan species (also with potential sialyl-Lewis X). Other candidates likely to contain the larger tri- and tetraantennary structures are kininogen-1 and histidine-rich glycoprotein (judged by the difference between apparent and calculated mass), but this has not been confirmed yet, possibly due to the technical difficulty associated with the analysis of these glycosylations.
When combining the contributions of the 24 major glycoproteins covered in this review to calculate a theoretical total plasma N-glycome, a remarkable congruence is observed with a TPNG profile registered for N-glycans released from human plasma (Figs. 1 and 2). Truncated fucosylated diantennary structures, mono- and disialylated diantennaries, as well as tri- and tetraantennary structures and their relative fucosylation are roughly in the correct ratios when compared to MALDI-TOF-MS with sialic acid-linkage-specific stabilization. The differences between the theoretical and measured N-glycome could be due to variations in the sample types or origins used in each study, as we have seen that phenotypes could occur at different ratios among ethnicities, but also due to the approximations in the protein concentrations that are often values averaged from multiple papers, or even to the remaining low abundant glycoproteins not covered by this review, although they should only account for a few percent of the TPNG.
Typical reflectron positive mode MALDI-TOF-MS spectrum of the total N-glycosylation of pooled human plasma after enzymatic N-glycan release, ethyl esterification, and hydrophilic-interaction liquid chromatography (HILIC) enrichment [109]. Glycan species are assigned as [M+Na]+ on basis of the reviewed plasma structures. Where multiple options are possible, the most abundant has been used for assignment. Sialic acid orientation is on basis of observed mass after ethyl esterification, while the other linkages are presumed on basis of literature. For fucosylation, diantennary structures are reported to mostly carry an α1-6-linked fucose on the reducing end N-acetylglucosamine, while tri- and tetraantennary structures are reported to mostly have α1-3-linked antennary fucosylation in the form of Lewis X (or sialyl-Lewis X when the antenna carries an α2-3-linked sialic acid). For the tri- and tetraantennary structures, antennae representation has been simplified for readability purposes
In all, we expect the knowledge gathered in this review to facilitate the clinical interpretation of plasma-wide glycosylation analysis. This review underlines the necessity for further protein-specific glycosylation analysis to fill the still considerable gaps in our understanding.
References
Opdenakker, G., Rudd, P.M., Ponting, C.P., Dwek, R.: Concepts and principles of glycobiology. FASEB J. 7(14), 1330–1337 (1993)
Rudd, P.M., Elliott, T., Cresswell, P., Wilson, I.A., Dwek, R.A.: Glycosylation and the immune system. Science 291(5512), 2370–2376 (2001)
Taniguchi, N.: Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. Biochem. Mol. Biol. Rep. 44(12), 772–781 (2011)
Sperandio, M., Gleissner, C.A., Ley, K.: Glycosylation in immune cell trafficking. Immunol. Rev. 230(1), 97–113 (2009)
Chen, Y., Hojo, S., Matsumoto, N., Yamamoto, K.: Regulation of Mac-2BP secretion is mediated by its N-glycan binding to ERGIC-53. Glycobiology 23(7), 904–916 (2013)
Varki, A., Cummings, R., Esko, J., Freeze, H., Stanley, P., Bertozzi, C., Hart, G., Etzler, M.: Essentials of Glycobiology, 2nd edn. Cold Spring Harbor Laboratory Press, New York (2009)
Tams, J.W., Vind, J., Welinder, K.G.: Adapting protein solubility by glycosylation: N-glycosylation mutants of coprinus cinereus peroxidase in salt and organic solutions. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1432(2), 214–221 (1999)
Nagae, M., Morita-Matsumoto, K., Arai, S., Wada, I., Matsumoto, Y., Saito, K., Hashimoto, Y., Yamaguchi, Y.: Structural change of N-glycan exposes hydrophobic surface of human transferrin. Glycobiology (2014)
Varki, A.: Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3(2), 97–130 (1993)
Molinari, M.: N-glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 3(6), 313–320 (2007)
Harazono, A., Kawasaki, N., Kawanishi, T., Hayakawa, T.: Site-specific glycosylation analysis of human apolipoprotein B100 using LC/ESI MS/MS. Glycobiology 15(5), 447–462 (2005)
Hulsmeier, A.J., Paesold-Burda, P., Hennet, T.: N-glycosylation site occupancy in serum glycoproteins using multiple reaction monitoring liquid chromatography-mass spectrometry. Mol. Cell. Proteomics 6(12), 2132–2138 (2007)
Chandler, K.B., Pompach, P., Goldman, R., Edwards, N.: Exploring site-specific N-glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. J. Proteome Res. 12(8), 3652–3666 (2013)
Huffman, J.E., Knezevic, A., Vitart, V., Kattla, J., Adamczyk, B., Novokmet, M., Igl, W., Pucic, M., Zgaga, L., Johannson, A., Redzic, I., Gornik, O., Zemunik, T., Polasek, O., Kolcic, I., Pehlic, M., Koeleman, C.A., Campbell, S., Wild, S.H., Hastie, N.D., Campbell, H., Gyllensten, U., Wuhrer, M., Wilson, J.F., Hayward, C., Rudan, I., Rudd, P.M., Wright, A.F., Lauc, G.: Polymorphisms in B3GAT1, SLC9A9 and MGAT5 are associated with variation within the human plasma N-glycome of 3533 European adults. Hum. Mol. Genet. 20(24), 5000–5011 (2011)
Ohtsubo, K., Marth, J.D.: Glycosylation in cellular mechanisms of health and disease. Cell 126(5), 855–867 (2006)
Thaysen-Andersen, M., Packer, N.H.: Site-specific glycoproteomics confirms that protein structure dictates formation of N-glycan type, core fucosylation and branching. Glycobiology 22(11), 1440–1452 (2012)
Wei, T., Liu, Q., He, F., Zhu, W., Hu, L., Guo, L., Zhang, J.: The role of N-acetylglucosaminyltransferases V in the malignancy of human hepatocellular carcinoma. Exp. Mol. Pathol. 93(1), 8–17 (2012)
Gornik, O., Wagner, J., Pučić, M., Knežević, A., Redžić, I., Lauc, G.: Stability of N-glycan profiles in human plasma. Glycobiology 19(12), 1547–1553 (2009)
Dall’Olio, F., Vanhooren, V., Chen, C.C., Slagboom, P.E., Wuhrer, M., Franceschi, C.: N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing Res. Rev. 12(2), 685–698 (2013)
Kristic, J., Vuckovic, F., Menni, C., Klaric, L., Keser, T., Beceheli, I., Pucic-Bakovic, M., Novokmet, M., Mangino, M., Thaqi, K., Rudan, P., Novokmet, N., Sarac, J., Missoni, S., Kolcic, I., Polasek, O., Rudan, I., Campbell, H., Hayward, C., Aulchenko, Y., Valdes, A., Wilson, J.F., Gornik, O., Primorac, D., Zoldos, V., Spector, T., Lauc, G.: Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. Ser. A Biol. Med. Sci. 69(7), 779–789 (2014)
Lauc, G., Huffman, J.E., Pučić, M., Zgaga, L., Adamczyk, B., Mužinić, A., Novokmet, M., Polašek, O., Gornik, O., Krištić, J., Keser, T., Vitart, V., Scheijen, B., Uh, H.-W., Molokhia, M., Patrick, A.L., McKeigue, P., Kolčić, I., Lukić, I.K., Swann, O., van Leeuwen, F.N., Ruhaak, L.R., Houwing-Duistermaat, J.J., Slagboom, P.E., Beekman, M., de Craen, A.J.M., Deelder, A.M., Zeng, Q., Wang, W., Hastie, N.D., Gyllensten, U., Wilson, J.F., Wuhrer, M., Wright, A.F., Rudd, P.M., Hayward, C., Aulchenko, Y., Campbell, H., Rudan, I.: Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9(1), e1003225 (2013)
Kobata, A.: Glycobiology in the field of aging research--introduction to glycogerontology. Biochimie 85(1–2), 13–24 (2003)
Ruhaak, L.R., Uh, H.W., Beekman, M., Hokke, C.H., Westendorp, R.G., Houwing-Duistermaat, J., Wuhrer, M., Deelder, A.M., Slagboom, P.E.: Plasma protein N-glycan profiles are associated with calendar age, familial longevity and health. J. Proteome Res. 10(4), 1667–1674 (2011)
Huhn, C., Selman, M.H., Ruhaak, L.R., Deelder, A.M., Wuhrer, M.: IgG glycosylation analysis. Proteomics 9(4), 882–913 (2009)
Adamczyk, B., Tharmalingam, T., Rudd, P.M.: Glycans as cancer biomarkers. Biochim. Biophys. Acta 1820(9), 1347–1353 (2012)
Freeze, H.H.: Genetic defects in the human glycome. Nat. Rev. Genet. 7(7), 537–551 (2006)
Jaeken, J., Matthijs, G.: Congenital disorders of glycosylation: a rapidly expanding disease family. Annu. Rev. Genomics Hum. Genet. 8, 261–278 (2007)
Bondt, A., Selman, M.H., Deelder, A.M., Hazes, J.M., Willemsen, S.P., Wuhrer, M., Dolhain, R.J.: Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J. Proteome Res. 12(10), 4522–4531 (2013)
Weis, W., Brown, J.H., Cusack, S., Paulson, J.C., Skehel, J.J., Wiley, D.C.: Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333(6172), 426–431 (1988)
Shields, R.L., Lai, J., Keck, R., O’Connell, L.Y., Hong, K., Meng, Y.G., Weikert, S.H.A., Presta, L.G.: Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277(30), 26733–26740 (2002)
Audfray, A., Varrot, A., Imberty, A.: Bacteria love our sugars: interaction between soluble lectins and human fucosylated glycans, structures, thermodynamics and design of competing glycocompounds. C. R. Chim. 16(5), 482–490 (2013)
Chen, K., Xu, W., Wilson, M., He, B., Miller, N.W., Bengten, E., Edholm, E.S., Santini, P.A., Rath, P., Chiu, A., Cattalini, M., Litzman, J., Bussel, J., Huang, B., Meini, A., Riesbeck, K., Cunningham-Rundles, C., Plebani, A., Cerutti, A.: Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 10(8), 889–898 (2009)
Nimmerjahn, F., Ravetch, J.V.: Anti-inflammatory actions of intravenous immunoglobulin. Annu. Rev. Immunol. 26, 513–533 (2008)
Huttenhain, R., Surinova, S., Ossola, R., Sun, Z., Campbell, D., Cerciello, F., Schiess, R., Bausch-Fluck, D., Rosenberger, G., Chen, J.C., Rinner, O., Kusebauch, U., Hajduch, M., Moritz, R.L., Wollscheid, B., Aebersold, R.: N-glycoprotein SRMAtlas a resource of mass spectrometric assays for N-glycosites enabling consistent and multiplexed protein quantification for clinical applications. Mol. Cell. Proteomics 12(4), 1005–1016 (2013)
Dalziel, M., Crispin, M., Scanlan, C.N., Zitzmann, N., Dwek, R.A.: Emerging principles for the therapeutic exploitation of glycosylation. Science 343(6166), 1235681 (2014)
Larkin, A., Imperiali, B.: The expanding horizons of asparagine-linked glycosylation. Biochemistry 50(21), 4411–4426 (2011)
Marino, K., Bones, J., Kattla, J.J., Rudd, P.M.: A systematic approach to protein glycosylation analysis: a path through the maze. Nat. Chem. Biol. 6(10), 713–723 (2010)
Bladergroen, M.R., Reiding, K.R., Hipgrave-Ederveen, A.L., Vreeker, G.C., Clerc, F., Holst, S., Bondt, A., Wuhrer, M., van der Burgt, Y.E.: Automation of high-throughput mass spectrometry-based plasma N-glycome analysis with linkage-specific sialic acid esterification. J. Proteome Res. (2015)
Stockmann, H., Duke, R.M., Millan Martin, S., Rudd, P.M.: Ultrahigh throughput, ultrafiltration-based N-glycomics platform for ultraperformance liquid chromatography (ULTRA(3)). Anal. Chem. 87(16), 8316–8322 (2015)
Klein, A.: Human total serum N-glycome. Adv. Clin. Chem. 46, 51–85 (2008)
Van Slyke, D.D., Hiller, A., Phillips, R.A., Hamilton, P.B., Dole, V.P., Archibald, R.M., Eder, H.A.: The estimation of plasma protein concentration from plasma specific gravity. J. Biol. Chem. 183, 331–347 (1950)
Dill, D., Costill, D.L.: Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 37(2), 247–248 (1974)
Peters Jr, T.: All about albumin: biochemistry, genetics, and medical applications. Academic Press, (1995)
Schmid, K., Kaufmann, H., Isemura, S., Bauer, F., Emura, J., Motoyama, T., Ishiguro, M., Nanno, S.: Structure of α1-acid glycoprotein. Complete amino acid sequence, multiple amino acid substitutions, and homology with the immunoglobulins. Biochemistry 12(14), 2711–2724 (1973)
Dage, J.L., Ackermann, B.L., Halsall, H.B.: Site localization of sialyl Lewisx antigen on α1-acid glycoprotein by high performance liquid chromatography-electrospray mass spectrometry. Glycobiology 8(8), 755–760 (1998)
Imre T, Schlosser G, Pocsfalvi G, Siciliano R, Molnár-Szöllosi E, Kremmer T, Malorni A, Vékey K. Glycosylation site analysis of human alpha-1-acid glycoprotein (AGP) by capillary liquid chromatography-electrospray mass spectrometry. J Mass Spectrom. 2005 Nov;40(11):1472--1483
Van Dijk, W., Havenaar, E., Brinkman-Van der Linden, E.: α1-acid glycoprotein (orosomucoid): pathophysiological changes in glycosylation in relation to its function. Glycoconj. J. 12(3), 227–233 (1995)
Brinkman-van der Linden, E.C., van Ommen, E.C., van Dijk, W.: Glycosylation of alpha 1-acid glycoprotein in septic shock: changes in degree of branching and in expression of sialyl Lewis(x) groups. Glycoconj. J. 13(1), 27–31 (1996)
Havenaar, E.C., Axford, J.S., Brinkman-van der Linden, E.C., Alavi, A., Van Ommen, E.C., van het Hof, B., Spector, T., Mackiewicz, A., Van Dijk, W.: Severe rheumatoid arthritis prohibits the pregnancy-induced decrease in alpha3-fucosylation of alpha1-acid glycoprotein. Glycoconj. J. 15(7), 723–729 (1998)
Wieruszeski, J.M., Fournet, B., Konan, D., Biou, D., Durand, G.: 400-MHz 1H-NMR spectroscopy of fucosylated tetrasialyl oligosaccharides isolated from normal and cirrhotic α1-acid glycoprotein. FEBS Lett 238(2), 390–394 (1988)
Ryden, I., Pahlsson, P., Lundblad, A., Skogh, T.: Fucosylation of alpha1-acid glycoprotein (orosomucoid) compared with traditional biochemical markers of inflammation in recent onset rheumatoid arthritis. Clin. Chim. Acta 317(1–2), 221–229 (2002)
Carrell, R.W., Jeppsson, J.O., Laurell, C.B., Brennan, S.O., Owen, M.C., Vaughan, L., Boswell, D.R.: Structure and variation of human alpha-1-antitrypsin. Nature 298(5872), 329–334 (1982)
Mega, T., Lujan, E., Yoshida, A.: Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. I. Isolation of glycopeptides. J. Biol. Chem. 255(9), 4053–4056 (1980)
Kolarich, D., Weber, A., Turecek, P.L., Schwarz, H.-P., Altmann, F.: Comprehensive glyco-proteomic analysis of human α1-antitrypsin and its charge isoforms. Proteomics 6(11), 3369–3380 (2006)
Mills, K., Mills, P.B., Clayton, P.T., Johnson, A.W., Whitehouse, D.B., Winchester, B.G.: Identification of alpha(1)-antitrypsin variants in plasma with the use of proteomic technology. Clin. Chem. 47(11), 2012–2022 (2001)
Ruhaak, L.R., Koeleman, C.A., Uh, H.-W., Stam, J.C., van Heemst, D., Maier, A.B., Houwing-Duistermaat, J.J., Hensbergen, P.J., Slagboom, P.E., Deelder, A.M.: Targeted biomarker discovery by high throughput glycosylation profiling of human plasma alpha1-antitrypsin and immunoglobulin A. PLoS One 8(9), e73082 (2013)
Mills, K., Mills, P.B., Clayton, P.T., Mian, N., Johnson, A.W., Winchester, B.G.: The underglycosylation of plasma alpha(1)-antitrypsin in congenital disorders of glycosylation type I is not random. Glycobiology 13(2), 73–85 (2003)
Ishioka, N., Takahashi, N., Putnam, F.W.: Amino acid sequence of human plasma alpha 1B-glycoprotein: homology to the immunoglobulin supergene family. Proc. Natl. Acad. Sci. U. S. A. 83(8), 2363–2367 (1986)
Bunkenborg, J., Pilch, B.J., Podtelejnikov, A.V., Wisniewski, J.R.: Screening for N-glycosylated proteins by liquid chromatography mass spectrometry. Proteomics 4(2), 454–465 (2004)
Liu, T., Qian, W.-J., Gritsenko, M.A., Camp, D.G., Monroe, M.E., Moore, R.J., Smith, R.D.: Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry. J. Proteome Res. 4(6), 2070–2080 (2005)
Huang, J., Lee, H., Zivkovic, A.M., Smilowitz, J.T., Rivera, N., German, J.B., Lebrilla, C.B.: Glycomic analysis of high density lipoprotein shows a highly sialylated particle. J. Proteome Res. 13(2), 681–691 (2014)
Nilsson, J., Rüetschi, U., Halim, A., Hesse, C., Carlsohn, E., Brinkmalm, G., Larson, G.: Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 6(11), 809–811 (2009)
Yoshioka, Y., Gejyo, F., Marti, T., Rickli, E.E., Burgi, W., Offner, G.D., Troxler, R.F., Schmid, K.: The complete amino acid sequence of the A-chain of human plasma alpha 2HS-glycoprotein. J. Biol. Chem. 261(4), 1665–1676 (1986)
Gejyo, F., Chang, J.L., Burgi, W., Schmid, K., Offner, G.D., Troxler, R.F., Van Halbeek, H., Dorland, L., Gerwig, G.J., Vliegenthart, J.F.: Characterization of the B-chain of human plasma alpha 2HS-glycoprotein. The complete amino acid sequence and primary structure of its heteroglycan. J. Biol. Chem. 258(8), 4966–4971 (1983)
Watzlawick, H., Walsh, M.T., Yoshioka, Y., Schmid, K., Brossmer, R.: Structure of the N- and O-glycans of the A-chain of human plasma alpha 2HS-glycoprotein as deduced from the chemical compositions of the derivatives prepared by stepwise degradation with exoglycosidases. Biochemistry 31(48), 12198–12203 (1992)
Wilson, N.L., Schulz, B.L., Karlsson, N.G., Packer, N.H.: Sequential analysis of N- and O-linked glycosylation of 2D-PAGE separated glycoproteins. J. Proteome Res. 1(6), 521–529 (2002)
Jia, W., Lu, Z., Fu, Y., Wang, H.P., Wang, L.H., Chi, H., Yuan, Z.F., Zheng, Z.B., Song, L.N., Han, H.H., Liang, Y.M., Wang, J.L., Cai, Y., Zhang, Y.K., Deng, Y.L., Ying, W.T., He, S.M., Qian, X.H.: A strategy for precise and large scale identification of core fucosylated glycoproteins. Mol. Cell. Proteomics 8(5), 913–923 (2009)
Kontro, H., Joenvaara, S., Haglund, C., Renkonen, R.: Comparison of sialylated N-glycopeptide levels in serum of pancreatic cancer patients, acute pancreatitis patients, and healthy controls. Proteomics 14(15), 1713–1723 (2014)
Sottrup-Jensen, L., Stepanik, T.M., Kristensen, T., Wierzbicki, D.M., Jones, C.M., Lonblad, P.B., Magnusson, S., Petersen, T.E.: Primary structure of human alpha 2-macroglobulin. V. The complete structure. J. Biol. Chem. 259(13), 8318–8327 (1984)
Chen, R., Jiang, X., Sun, D., Han, G., Wang, F., Ye, M., Wang, L., Zou, H.: Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J. Proteome Res. 8(2), 651–661 (2009)
Lin, Z., Lo, A., Simeone, D.M., Ruffin, M.T., Lubman, D.M.: An N-glycosylation analysis of human alpha-2-macroglobulin using an integrated approach. J. Proteomics Bioinf. 5, 127 (2012)
Arnold, J.N., Wallis, R., Willis, A.C., Harvey, D.J., Royle, L., Dwek, R.A., Rudd, P.M., Sim, R.B.: Interaction of mannan binding lectin with alpha2 macroglobulin via exposed oligomannose glycans: a conserved feature of the thiol ester protein family? J. Biol. Chem. 281(11), 6955–6963 (2006)
Panzironi, C., Silvestrini, B., Mo, M.Y., Lahita, R., Mruk, D., Cheng, C.Y.: An increase in the carbohydrate moiety of alpha 2-macroglobulin is associated with systemic lupus erythematosus (SLE). Biochem. Mol. Biol. Int. 43(6), 1305–1322 (1997)
Gunnarsson, M., Stigbrand, T., Jensen, P.E.: Aberrant forms of alpha(2)-macroglobulin purified from patients with multiple sclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 295(1–2), 27–40 (2000)
Demelbauer, U.M., Plematl, A., Kremser, L., Allmaier, G., Josic, D., Rizzi, A.: Characterization of glyco isoforms in plasma-derived human antithrombin by on-line capillary zone electrophoresis-electrospray ionization-quadrupole ion trap-mass spectrometry of the intact glycoproteins. Electrophoresis 25(13), 2026–2032 (2004)
Demelbauer, U.M., Plematl, A., Josic, D., Allmaier, G., Rizzi, A.: On the variation of glycosylation in human plasma derived antithrombin. J. Chromatogr. A 1080(1), 15–21 (2005)
Turk, B., Brieditis, I., Bock, S.C., Olson, S.T., Bjork, I.: The oligosaccharide side chain on Asn-135 of alpha-antithrombin, absent in beta-antithrombin, decreases the heparin affinity of the inhibitor by affecting the heparin-induced conformational change. Biochemistry 36(22), 6682–6691 (1997)
Peterson, C.B., Blackburn, M.N.: Isolation and characterization of an antithrombin III variant with reduced carbohydrate content and enhanced heparin binding. J. Biol. Chem. 260(1), 610–615 (1985)
Kasturi, L., Eshleman, J.R., Wunner, W.H., Shakin-Eshleman, S.H.: The hydroxy amino acid in an Asn-X-Ser/Thr sequon can influence N-linked core glycosylation efficiency and the level of expression of a cell surface glycoprotein. J. Biol. Chem. 270(24), 14756–14761 (1995)
Picard, V., Ersdal-Badju, E., Bock, S.C.: Partial glycosylation of antithrombin III asparagine-135 is caused by the serine in the third position of its N-glycosylation consensus sequence and is responsible for production of the beta-antithrombin III isoform with enhanced heparin affinity. Biochemistry 34(26), 8433–8440 (1995)
Pol-Fachin, L., Franco Becker, C., Almeida Guimarães, J., Verli, H.: Effects of glycosylation on heparin binding and antithrombin activation by heparin. Proteins 79(9), 2735–2745 (2011)
Franzén, L.E., Svensson, S., Larm, O.: Structural studies on the carbohydrate portion of human antithrombin III. J. Biol. Chem. 255(11), 5090–5093 (1980)
Mizuochi, T., Fujii, J., Kurachi, K., Kobata, A.: Structural studies of the carbohydrate moiety of human antithrombin III. Arch. Biochem. Biophys. 203(1), 458–465 (1980)
Demelbauer, U.M., Zehl, M., Plematl, A., Allmaier, G., Rizzi, A.: Determination of glycopeptide structures by multistage mass spectrometry with low-energy collision-induced dissociation: comparison of electrospray ionization quadrupole ion trap and matrix-assisted laser desorption/ionization quadrupole ion trap reflectron time-of-flight approaches. Rapid Commun. Mass Spectrom. 18(14), 1575–1582 (2004)
Plematl, A., Demelbauer, U.M., Josic, D., Rizzi, A.: Determination of the site-specific and isoform-specific glycosylation in human plasma-derived antithrombin by IEF and capillary HPLC-ESI-MS/MS. Proteomics 5(15), 4025–4033 (2005)
Martínez-Martínez, I., Ordóñez, A., Navarro-Fernández, J., Pérez-Lara, A., Gutiérrez-Gallego, R., Giraldo, R., Martínez, C., Llop, E., Vicente, V., Corral, J.: Antithrombin Murcia (K241E) causing antithrombin deficiency: a possible role for altered glycosylation. Haematologica 95(8), 1358–1365 (2010)
Garner, B., Harvey, D.J., Royle, L., Frischmann, M., Nigon, F., Chapman, M.J., Rudd, P.M.: Characterization of human apolipoprotein B100 oligosaccharides in LDL subfractions derived from normal and hyperlipidemic plasa: deficiency of alpha-N-acetylneuraminyllactosyl-ceramide in light and small dense LDL particles. Glycobiology 11(10), 791–802 (2001)
Attie, A.D., Weinstein, D.B., Freeze, H.H., Pittman, R.C., Steinberg, D.: Unaltered catabolism of desialylated low-density lipoprotein in the pig and in cultured rat hepatocytes. Biochem. J. 180(3), 647–654 (1979)
Filipovic, I., Schwarzmann, G., Mraz, W., Wiegandt, H., Buddecke, E.: Sialic-acid content of low-density lipoproteins controls their binding and uptake by cultured-cells. Eur. J. Biochem. 93(1), 51–55 (1979)
Fujioka, Y., Taniguchi, T., Ishikawa, Y., Yokoyama, M.: Significance of acidic sugar chains of apolipoprotein B-100 in cellular metabolism of low-density lipoproteins. J. Lab. Clin. Med. 136(5), 355–362 (2000)
Schindler, P.A., Settineri, C.A., Collet, X., Fielding, C.J., Burlingame, A.L.: Site-specific detection and structural characterization of the glycosylation of human plasma proteins lecithin: cholesterol acyltransferase and apolipoprotein D using HPLC/electrospray mass spectrometry and sequential glycosidase digestion. Protein Sci. 4(4), 791–803 (1995)
Zeng, C.H., Spielman, A.I., Vowels, B.R., Leyden, J.J., Biemann, K., Preti, G.: A human axillary odorant is carried by apolipoprotein D. Proc. Natl. Acad. Sci. U. S. A. 93(13), 6626–6630 (1996)
Kobata, A.: Use of endo-and exoglycosidases for structural studies of glycoconjugates. Anal. Biochem. 100(1), 1–14 (1979)
Morton, R.E., Gnizak, H.M., Greene, D.J., Cho, K.H., Paromov, V.M.: Lipid transfer inhibitor protein (apolipoprotein F) concentration in normolipidemic and hyperlipidemic subjects. J. Lipid Res. 49(1), 127–135 (2008)
Lagor, W.R., Brown, R.J., Toh, S.A., Millar, J.S., Fuki, I.V., de la Llera-Moya, M., Yuen, T., Rothblat, G., Billheimer, J.T., Rader, D.J.: Overexpression of apolipoprotein F reduces HDL cholesterol levels in vivo. Arterioscler. Thromb. Vasc. Biol. 29(1), 40–46 (2009)
Halim, A., Nilsson, J., Ruetschi, U., Hesse, C., Larson, G.: Human urinary glycoproteomics; attachment site specific analysis of N- and O-linked glycosylations by CID and ECD. Mol. Cell. Proteomics 11(4), M111 013649 (2012)
Lozier, J., Takahashi, N., Putnam, F.W.: Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc. Natl. Acad. Sci. U. S. A. 81(12), 3640–3644 (1984)
De Groot, P.G.: β2-Glycoprotein I: evolution, structure and function. J. Thromb. Haemost. 9(7), 1275–1284 (2011)
Kondo, A., Miyamoto, T., Yonekawa, O., Giessing, A.M., Østerlund, E.C., Jensen, O.N.: Glycopeptide profiling of beta-2-glycoprotein I by mass spectrometry reveals attenuated sialylation in patients with antiphospholipid syndrome. J. Proteome 73(1), 123–133 (2009)
Bouma, B., de Groot, P.G., van den Elsen, J.M.H., Ravelli, R.B.G., Schouten, A., Simmelink, M.J.A., Derksen, R.H.W.M., Kroon, J., Gros, P.: Adhesion mechanism of human β2‐glycoprotein I to phospholipids based on its crystal structure. 18(19), (1999)
Kristensen, T., Schousboe, I., Boel, E., Mulvihill, E.M., Hansen, R.R., Møller, K.B., Møller, N.P.H., Sottrup-Jensen, L.: Molecular cloning and mammalian expression of human β 2-glycoprotein I cDNA. FEBS Lett 289(2), 183–186 (1991)
Powell, A.K., Harvey, D.J.: Stabilization of sialic acids in N-linked oligosaccharides and gangliosides for analysis by positive ion matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 10(9), 1027–1032 (1996)
Takahashi, N., Ortel, T.L., Putnam, F.W.: Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc. Natl. Acad. Sci. U. S. A. 81(2), 390–394 (1984)
Endo, M., Suzuki, K., Schmid, K., Fournet, B., Karamanos, Y., Montreuil, J., Dorland, L., van Halbeek, H., Vliegenthart, J.F.: The structures and microheterogeneity of the carbohydrate chains of human plasma ceruloplasmin. A study employing 500-MHz 1H-NMR spectroscopy. J. Biol. Chem. 257(15), 8755–8760 (1982)
Harazono, A., Kawasaki, N., Itoh, S., Hashii, N., Ishii-Watabe, A., Kawanishi, T., Hayakawa, T.: Site-specific N-glycosylation analysis of human plasma ceruloplasmin using liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Biochem. 348(2), 259–268 (2006)
Weisel, J.W.: Fibrinogen and fibrin. In: David, A.D.P., John, M.S. (eds.) Advances in Protein Chemistry, vol. 70, pp. 247–299. Academic Press, (2005)
Zauner, G., Hoffmann, M., Rapp, E., Koeleman, C.A.M., Dragan, I., Deelder, A.M., Wuhrer, M., Hensbergen, P.J.: Glycoproteomic analysis of human fibrinogen reveals novel regions of O-glycosylation. J. Proteome Res. 11(12), 5804–5814 (2012)
Lewandrowski, U., Moebius, J., Walter, U., Sickmann, A.: Elucidation of N-glycosylation sites on human platelet proteins: a glycoproteomic approach. Mol. Cell. Proteomics 5(2), 226–233 (2006)
Reiding, K.R., Blank, D., Kuijper, D.M., Deelder, A.M., Wuhrer, M.: High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal. Chem. 86(12), 5784–5793 (2014)
Adamczyk, B., Struwe, W.B., Ercan, A., Nigrovic, P.A., Rudd, P.M.: Characterization of fibrinogen glycosylation and its importance for serum/plasma N-glycome analysis. J. Proteome Res. 12(1), 444–454 (2012)
Martinez, J., Keane, P.M., Gilman, P.B., Palascak, J.E.: The abnormal carbohydrate-composition of the dysfibrinogenemia associated with liver-disease. Ann. N. Y. Acad. Sci. 408(Jun), 388–396 (1983)
Dang, C.V., Shin, C.K., Bell, W.R., Nagaswami, C., Weisel, J.W.: Fibrinogen sialic-acid residues are low affinity calcium-binding sites that influence fibrin assembly. J. Biol. Chem. 264(25), 15104–15108 (1989)
Langer, B.G., Weisel, J.W., Dinauer, P.A., Nagaswami, C., Bell, W.R.: Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J. Biol. Chem. 263(29), 15056–15063 (1988)
Woodhead, J.L., Nagaswami, C., Matsuda, M., Arocha-Pinango, C.L., Weisel, J.W.: The ultrastructure of fibrinogen Caracas II molecules, fibers, and clots. J. Biol. Chem. 271(9), 4946–4953 (1996)
Sugo, T., Nakamikawa, C., Takano, H., Mimuro, J., Yamaguchi, S., Mosesson, M.W., Meh, D.A., DiOrio, J.P., Takahashi, N., Takahashi, H., Nagai, K., Matsuda, M.: Fibrinogen Niigata with impaired fibrin assembly: an inherited dysfibrinogen with a Bbeta Asn-160 to Ser substitution associated with extra glycosylation at Bbeta Asn-158. Blood 94(11), 3806–3813 (1999)
Yamazumi, K., Shimura, K., Terukina, S., Takahashi, N., Matsuda, M.: A gamma methionine-310 to threonine substitution and consequent N-glycosylation at gamma asparagine-308 identified in a congenital dysfibrinogenemia associated with posttraumatic bleeding, fibrinogen Asahi. J. Clin. Invest. 83(5), 1590–1597 (1989)
Sugo, T., Sekine, O., Nakamikawa, C., Endo, H., Arocha-Pinango, C.L., Matsuda, M.: Mode of perturbation of Asahi fibrin assembly by the extra oligosaccharides. Ann. N. Y. Acad. Sci. 936, 223–225 (2001)
Langlois, M.R., Delanghe, J.R.: Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 42(10), 1589–1600 (1996)
Zhang, S., Jiang, K., Sun, C., Lu, H., Liu, Y.: Quantitative analysis of site-specific N-glycans on sera haptoglobin β chain in liver diseases. Acta Biochim. Biophys. Sin. 45(12), 1021–1029 (2013)
Pompach, P., Brnakova, Z., Sanda, M., Wu, J., Edwards, N., Goldman, R.: Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Mol. Cell. Proteomics 12(5), 1281–1293 (2013)
Pompach, P., Ashline, D.J., Brnakova, Z., Benicky, J., Sanda, M., Goldman, R.: Protein and site specificity of fucosylation in liver-secreted glycoproteins. J. Proteome Res. 13(12), 5561–5569 (2014)
Wang, D., Hincapie, M., Rejtar, T., Karger, B.L.: Ultrasensitive characterization of site-specific glycosylation of affinity-purified haptoglobin from lung cancer patient plasma using 10 μm i.d. Porous layer open tubular liquid chromatography–linear ion trap collision-induced dissociation/electron transfer dissociation mass spectrometry. Anal. Chem. 83(6), 2029–2037 (2011)
Nakano, M., Nakagawa, T., Ito, T., Kitada, T., Hijioka, T., Kasahara, A., Tajiri, M., Wada, Y., Taniguchi, N., Miyoshi, E.: Site-specific analysis of N-glycans on haptoglobin in sera of patients with pancreatic cancer: a novel approach for the development of tumor markers. Int. J. Cancer 122(10), 2301–2309 (2008)
Zhu, J., Lin, Z., Wu, J., Yin, H., Dai, J., Feng, Z., Marrero, J., Lubman, D.M.: Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J. Proteome Res. 13(6), 2986–2997 (2014)
Carlsson, M.C., Cederfur, C., Schaar, V., Balog, C., Lepur, A., Touret, F., Salomonsson, E., Deelder, A.M., Fernö, M., Olsson, H.: Galectin-1-binding glycoforms of haptoglobin with altered intracellular trafficking, and increase in metastatic breast cancer patients. PLoS One 6(10), e26560 (2011)
Carlsson, M.C., Balog, C.I.A., Kilsgård, O., Hellmark, T., Bakoush, O., Segelmark, M., Fernö, M., Olsson, H., Malmström, J., Wuhrer, M., Leffler, H.: Different fractions of human serum glycoproteins bind galectin-1 or galectin-8, and their ratio may provide a refined biomarker for pathophysiological conditions in cancer and inflammatory disease. Biochim. Biophys. Acta Gen. Subj. 1820(9), 1366–1372 (2012)
Takahashi, N., Takahashi, Y., Putnam, F.W.: Structure of human hemopexin: O-glycosyl and N-glycosyl sites and unusual clustering of tryptophan residues. Proc. Natl. Acad. Sci. U. S. A. 81(7), 2021–2025 (1984)
Kristiansen, T.Z., Bunkenborg, J., Gronborg, M., Molina, H., Thuluvath, P.J., Argani, P., Goggins, M.G., Maitra, A., Pandey, A.: A proteomic analysis of human bile. Mol. Cell. Proteomics 3(7), 715–728 (2004)
Ramachandran, P., Boontheung, P., Xie, Y., Sondej, M., Wong, D.T., Loo, J.A.: Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J. Proteome Res. 5(6), 1493–1503 (2006)
Frantikova, V., Borvak, J., Kluh, I., Moravek, L.: Amino acid sequence of the N-terminal region of human hemopexin. FEBS Lett 178(2), 213–216 (1984)
Lee, J.H., Cho, C.H., Kim, S.H., Kang, J.G., Yoo, J.S., Chang, C.L., Ko, J.H., Kim, Y.S.: Semi-quantitative measurement of a specific glycoform using a DNA-tagged antibody and lectin affinity chromatography for glyco-biomarker development. Mol. Cell. Proteomics 14(3), 782–795 (2015)
Yin, H., Lin, Z., Nie, S., Wu, J., Tan, Z., Zhu, J., Dai, J., Feng, Z., Marrero, J., Lubman, D.M.: Mass-selected site-specific core-fucosylation of ceruloplasmin in alcohol-related hepatocellular carcinoma. J. Proteome Res. 13(6), 2887–2896 (2014)
Benicky, J., Sanda, M., Pompach, P., Wu, J., Goldman, R.: Quantification of fucosylated hemopexin and complement factor H in plasma of patients with liver disease. Anal. Chem. 86(21), 10716–10723 (2014)
Debruyne, E.N., Vanderschaeghe, D., Van Vlierberghe, H., Vanhecke, A., Callewaert, N., Delanghe, J.R.: Diagnostic value of the hemopexin N-glycan profile in hepatocellular carcinoma patients. Clin. Chem. 56(5), 823–831 (2010)
Lebreton, J.P., Joisel, F., Raoult, J.P., Lannuzel, B., Rogez, J.P., Humbert, G.: Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J. Clin. Invest. 64(4), 1118–1129 (1979)
Hennis, B.C., van Boheemen, P.A., Wakabayashi, S., Koide, T., Hoffmann, J.J., Kievit, P., Dooijewaard, G., Jansen, J.G., Kluft, C.: Identification and genetic analysis of a common molecular variant of histidine-rich glycoprotein with a difference of 2kD in apparent molecular weight. Thromb. Haemost. 74(6), 1491–1496 (1995)
Koide, T., Foster, D., Yoshitake, S., Davie, E.W.: Amino acid sequence of human histidine-rich glycoprotein derived from the nucleotide sequence of its cDNA. Biochemistry 25(8), 2220–2225 (1986)
Stenflo, J., Fernlund, P.: Amino acid sequence of the heavy chain of bovine protein C. J. Biol. Chem. 257(20), 12180–12190 (1982)
Lottspeich, F., Kellermann, J., Henschen, A., Foertsch, B., MüLler-Esterl, W.: The amino acid sequence of the light chain of human high-molecular-mass kiniogen. Eur. J. Biochem. 152(2), 307–314 (1985)
Bones, J., Byrne, J.C., O’Donoghue, N., McManus, C., Scaife, C., Boissin, H., Nastase, A., Rudd, P.M.: Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J. Proteome Res. 10(3), 1246–1265 (2011)
Leger, D., Campion, B., Decottignies, J.P., Montreuil, J., Spik, G.: Physiological significance of the marked increased branching of the glycans of human serotransferrin during pregnancy. Biochem. J. 257(1), 231–238 (1989)
Thorstensen, K., Romslo, I.: The role of transferrin in the mechanism of cellular iron uptake. Biochem. J. 271(1), 1–10 (1990)
Satomi, Y., Shimonishi, Y., Takao, T.: N-glycosylation at Asn(491) in the Asn-Xaa-Cys motif of human transferrin. FEBS Lett 576(1–2), 51–56 (2004)
Satomi, Y., Shimonishi, Y., Hase, T., Takao, T.: Site-specific carbohydrate profiling of human transferrin by nano-flow liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 18(24), 2983–2988 (2004)
Yamashita, K., Koide, N., Endo, T., Iwaki, Y., Kobata, A.: Altered glycosylation of serum transferrin of patients with hepatocellular-carcinoma. J. Biol. Chem. 264(5), 2415–2423 (1989)
van Rooijen, J.J.M., Jeschke, U., Kamerling, J.P., Vliegenthart, J.F.G.: Expression of N-linked sialyl Lex determinants and O-glycans in the carbohydrate moiety of human amniotic fluid transferrin during pregnancy. Glycobiology 8(11), 1053–1064 (1998)
Futakawa, S., Nara, K., Miyajima, M., Kuno, A., Ito, H., Kaji, H., Shirotani, K., Honda, T., Tohyama, Y., Hoshi, K., Hanzawa, Y., Kitazume, S., Imamaki, R., Furukawa, K., Tasaki, K., Arai, H., Yuasa, T., Abe, M., Arai, H., Narimatsu, H., Hashimoto, Y.: A unique N-glycan on human transferrin in CSF: a possible biomarker for iNPH. Neurobiol. Aging 33(8), 1807–1815 (2012)
Grünewald, S., Matthijs, G., Jaeken, J.: Congenital disorders of glycosylation: a review. Pediatr. Res. 52(5), 618–624 (2002)
Jaeken, J.: Congenital disorders of glycosylation. Ann. N. Y. Acad. Sci. 1214, 190–198 (2010)
Lefeber, D.J., Morava, E., Jaeken, J.: How to find and diagnose a CDG due to defective N-glycosylation. J. Inherit. Metab. Dis. 34(4), 849–852 (2011)
Rainio, J., Ahola, S., Kangastupa, P., Kultti, J., Tuomi, H., Karhunen, P.J., Helander, A., Niemelä, O.: Comparison of ethyl glucuronide and carbohydrate-deficient transferrin in different body fluids for post-mortem identification of alcohol use. Alcohol Alcohol. 49(1), 55–59 (2014)
Hwang, H., Lee, J.Y., Lee, H.K., Park, G.W., Jeong, H.K., Moon, M.H., Kim, J.Y., Yoo, J.S.: In-depth analysis of site-specific N-glycosylation in vitronectin from human plasma by tandem mass spectrometry with immunoprecipitation. Anal. Bioanal. Chem. 406(30), 7999–8011 (2014)
Ogawa, H., Yoneda, A., Seno, N., Hayashi, M., Ishizuka, I., Hase, S., Matsumoto, I.: Structures of the N‐linked oligosaccharides on human plasma vitronectin. Eur. J. Biochem. 230(3), 994–1000 (1995)
Lee, H.-J., Cha, H.-J., Lim, J.-S., Lee, S.H., Song, S.Y., Kim, H., Hancock, W.S., Yoo, J.S., Paik, Y.-K.: Abundance-ratio-based semiquantitative analysis of site-specific N-linked glycopeptides present in the plasma of hepatocellular carcinoma patients. J. Proteome Res. 13(5), 2328–2338 (2014)
Araki, T., Gejyo, F., Takagaki, K., Haupt, H., Schwick, H.G., Burgi, W., Marti, T., Schaller, J., Rickli, E., Brossmer, R., et al.: Complete amino acid sequence of human plasma Zn-alpha 2-glycoprotein and its homology to histocompatibility antigens. Proc. Natl. Acad. Sci. U. S. A. 85(3), 679–683 (1988)
Delker, S.L., West Jr., A.P., McDermott, L., Kennedy, M.W., Bjorkman, P.J.: Crystallographic studies of ligand binding by Zn-alpha2-glycoprotein. J. Struct. Biol. 148(2), 205–213 (2004)
Hassan, M.I., Bilgrami, S., Kumar, V., Singh, N., Yadav, S., Kaur, P., Singh, T.P.: Crystal structure of the novel complex formed between zinc alpha2-glycoprotein (ZAG) and prolactin-inducible protein (PIP) from human seminal plasma. J. Mol. Biol. 384(3), 663–672 (2008)
Sanchez, L.M., Chirino, A.J., Bjorkman, P.: Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science 283(5409), 1914–1919 (1999)
Arnold, J.N., Wormald, M.R., Sim, R.B., Rudd, P.M., Dwek, R.A.: The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 (2007)
Torano, A., Tsuzukida, Y., Liu, Y.S., Putnam, F.W.: Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc. Natl. Acad. Sci. U. S. A. 74(6), 2301–2305 (1977)
Mattu, T.S., Pleass, R.J., Willis, A.C., Kilian, M., Wormald, M.R., Lellouch, A.C., Rudd, P.M., Woof, J.M., Dwek, R.A.: The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J. Biol. Chem. 273(4), 2260–2272 (1998)
Tanaka, A., Iwase, H., Hiki, Y., Kokubo, T., Ishii-Karakasa, I., Toma, K., Kobayashi, Y., Hotta, K.: Evidence for a site-specific fucosylation of N-linked oligosaccharide of immunoglobulin A1 from normal human serum. Glycoconj. J. 15(10), 995–1000 (1998)
Klapoetke, S.C., Zhang, J., Becht, S.: Glycosylation characterization of human IgA1 with differential deglycosylation by UPLC-ESI TOF MS. J. Pharm. Biomed. Anal. 56(3), 513–520 (2011)
Gomes, M.M., Wall, S.B., Takahashi, K., Novak, J., Renfrow, M.B., Herr, A.B.: Analysis of IgA1 N-glycosylation and its contribution to FcαRI binding. Biochemistry 47(43), 11285–11299 (2008)
Monteiro, R.C., Van De Winkel, J.G.: IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 (2003)
Takayasu, T., Suzuki, S., Kametani, F., Takahashi, N., Shinoda, T., Okuyama, T., Munekata, E.: Amino acid sequence of galactosamine-containing glycopeptides in the hinge region of a human immunoglobulin D. Biochem. Biophys. Res. Commun. 105(3), 1066–1071 (1982)
Takahashi, N., Tetaert, D., Debuire, B., Lin, L.C., Putnam, F.W.: Complete amino acid sequence of the delta heavy chain of human immunoglobulin D. Proc. Natl. Acad. Sci. U. S. A. 79(9), 2850–2854 (1982)
Mellis, S.J., Baenziger, J.U.: Structures of the O-glycosidically linked oligosaccharides of human IgD. J. Biol. Chem. 258(19), 11557–11563 (1983)
Arnold, J.N., Radcliffe, C.M., Wormald, M.R., Royle, L., Harvey, D.J., Crispin, M., Dwek, R.A., Sim, R.B., Rudd, P.M.: The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J. Immunol. 173(11), 6831–6840 (2004)
Gala, F.A., Morrison, S.L.: The role of constant region carbohydrate in the assembly and secretion of human IgD and IgA1. J. Biol. Chem. 277(32), 29005–29011 (2002)
Dorrington, K.J., Bennich, H.H.: Structure-function relationships in human immunoglobulin E. Immunol. Rev. 41(1), 3–25 (1978)
Flanagan, J.G., Rabbitts, T.H.: The sequence of a human immunoglobulin epsilon heavy chain constant region gene, and evidence for three non-allelic genes. EMBO J. 1(5), 655–660 (1982)
Plomp, R., Hensbergen, P.J., Rombouts, Y., Zauner, G., Dragan, I., Koeleman, C.A.M., Deelder, A.M., Wuhrer, M.: Site-specific N-glycosylation analysis of human immunoglobulin E. J. Proteome Res. 13(2), 536–546 (2014)
Björklund, J.E.M., Karlsson, T., Magnusson, C.G.M.: N-glycosylation influences epitope expression and receptor binding structures in human IgE. Mol. Immunol. 36(3), 213–221 (1999)
Shade, K.-T.C., Platzer, B., Washburn, N., Mani, V., Bartsch, Y.C., Conroy, M., Pagan, J.D., Bosques, C., Mempel, T.R., Fiebiger, E., Anthony, R.M.: A single glycan on IgE is indispensable for initiation of anaphylaxis. J. Exp. Med. 212(4), 457–467 (2015)
Woof, J.M., Burton, D.R.: Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 4(2), 89–99 (2004)
Zauner, G., Selman, M.H., Bondt, A., Rombouts, Y., Blank, D., Deelder, A.M., Wuhrer, M.: Glycoproteomic analysis of antibodies. Mol. Cell. Proteomics 12(4), 856–865 (2013)
Maverakis, E., Kim, K., Shimoda, M., Gershwin, M.E., Patel, F., Wilken, R., Raychaudhuri, S., Ruhaak, L.R., Lebrilla, C.B.: Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J. Autoimmun. 57, 1–13 (2015)
Stadlmann, J., Pabst, M., Altmann, F.: Analytical and functional aspects of antibody sialylation. J. Clin. Immunol. 30(Suppl 1), 15–19 (2010)
Bondt, A., Rombouts, Y., Selman, M.H., Hensbergen, P.J., Reiding, K.R., Hazes, J.M., Dolhain, R.J., Wuhrer, M.: Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteomics 13(11), 3029–3039 (2014)
Dalziel, M., McFarlane, I., Axford, J.S.: Lectin analysis of human immunoglobulin G N-glycan sialylation. Glycoconj. J. 16(12), 801–807 (1999)
Wuhrer, M., Stam, J.C., van de Geijn, F.E., Koeleman, C.A.M., Verrips, C.T., Dolhain, R.J.E.M., Hokke, C.H., Deelder, A.M.: Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 7(22), 4070–4081 (2007)
Yuan, W., Sanda, M., Wu, J., Koomen, J., Goldman, R.: Quantitative analysis of immunoglobulin subclasses and subclass specific glycosylation by LC–MS–MRM in liver disease. J. Proteome 116, 24–33 (2015)
Plomp, R., Dekkers, G., Rombouts, Y., Visser, R., Koeleman, C.A., Kammeijer, G.S., Jansen, B.C., Rispens, T., Hensbergen, P.J., Vidarsson, G., Wuhrer, M.: Hinge-region O-glycosylation of human immunoglobulin G3 (IgG3). Mol. Cell. Proteomics 14(5), 1373–1384 (2015)
Schwab, I., Nimmerjahn, F.: Role of sialylation in the anti-inflammatory activity of intravenous immunoglobulin - F(ab’)(2) versus Fc sialylation. Clin. Exp. Immunol. 178(Suppl 1), 97–99 (2014)
Bohm, S., Kao, D., Nimmerjahn, F.: Sweet and sour: the role of glycosylation for the anti-inflammatory activity of immunoglobulin G. Curr. Top. Microbiol. Immunol. 382, 393–417 (2014)
Selman, M.H., McDonnell, L.A., Palmblad, M., Ruhaak, L.R., Deelder, A.M., Wuhrer, M.: Immunoglobulin G glycopeptide profiling by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 82(3), 1073–1081 (2010)
Parekh, R., Isenberg, D., Ansell, B., Roitt, I., Dwek, R., Rademacher, T.: Galactosylation of Igg associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet 331(8592), 966–969 (1988)
Parekh, R., Isenberg, D., Rook, G., Roitt, I., Dwek, R., Rademacher, T.: A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 2(2), 101–114 (1989)
Perdivara, I., Peddada, S.D., Miller, F.W., Tomer, K.B., Deterding, L.J.: Mass spectrometric determination of IgG subclass-specific glycosylation profiles in siblings discordant for myositis syndromes. J. Proteome Res. 10(7), 2969–2978 (2011)
Moore, J.S., Wu, X., Kulhavy, R., Tomana, M., Novak, J., Moldoveanu, Z., Brown, R., Goepfert, P.A., Mestecky, J.: Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19(4), 381–389 (2005)
Wuhrer, M., Stavenhagen, K., Koeleman, C.A., Selman, M.H., Harper, L., Jacobs, B.C., Savage, C.O., Jefferis, R., Deelder, A.M., Morgan, M.: Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J. Proteome Res. 14(4), 1657–1665 (2015)
Parekh, R.B., Dwek, R.A., Sutton, B.J., Fernandes, D.L., Leung, A., Stanworth, D., Rademacher, T.W., Mizuochi, T., Taniguchi, T., Matsuta, K., et al.: Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316(6027), 452–457 (1985)
Ferrara, C., Grau, S., Jager, C., Sondermann, P., Brunker, P., Waldhauer, I., Hennig, M., Ruf, A., Rufer, A.C., Stihle, M., Umana, P., Benz, J.: Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U. S. A. 108(31), 12669–12674 (2011)
Okazaki, A., Shoji-Hosaka, E., Nakamura, K., Wakitani, M., Uchida, K., Kakita, S., Tsumoto, K., Kumagai, I., Shitara, K.: Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J. Mol. Biol. 336(5), 1239–1249 (2004)
Raju, T.S.: Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20(4), 471–478 (2008)
Anthony, R.M., Nimmerjahn, F., Ashline, D.J., Reinhold, V.N., Paulson, J.C., Ravetch, J.V.: Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320(5874), 373–376 (2008)
Kibe, T., Fujimoto, S., Ishida, C., Togari, H., Wada, Y., Okada, S., Nakagawa, H., Tsukamoto, Y., Takahashi, N.: Glycosylation and placental transport of immunoglobulin G. J. Clin. Biochem. Nutr. 21(1), 57–63 (1996)
van de Geijn, F.E., Wuhrer, M., Selman, M.H., Willemsen, S.P., de Man, Y.A., Deelder, A.M., Hazes, J.M., Dolhain, R.J.: Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11(6), R193 (2009)
Selman, M.H., Derks, R.J., Bondt, A., Palmblad, M., Schoenmaker, B., Koeleman, C.A., van de Geijn, F.E., Dolhain, R.J., Deelder, A.M., Wuhrer, M.: Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. J. Proteome 75(4), 1318–1329 (2012)
Putnam, F.W., Florent, G., Paul, C., Shinoda, T., Shimizu, A.: Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science 182(4109), 287–291 (1973)
Arnold, J.N., Wormald, M.R., Suter, D.M., Radcliffe, C.M., Harvey, D.J., Dwek, R.A., Rudd, P.M., Sim, R.B.: Human serum Igm glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J. Biol. Chem. 280(32), 29080–29087 (2005)
Pabst, M., Kuster, S.K., Wahl, F., Krismer, J., Dittrich, P.S., Zenobi, R.: A microarray-MALDI-MS approach for site-specific protein N-glycosylation analysis, as demonstrated for human serum IgM. Mol. Cell. Proteomics (2015)
Kushner, I.: The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 389(1), 39–48 (1982)
Blain, P.G., Mucklow, J.C., Rawlins, M.D., Roberts, D.F., Routledge, P.A., Shand, D.G.: Determinants of plasma alpha 1-acid glycoprotein (AAG) concentrations in health. Br. J. Clin. Pharmacol. 20(5), 500–502 (1985)
Fournier, T., Medjoubi-N, N., Porquet, D.: Alpha-1-acid glycoprotein. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1482(1), 157–171 (2000)
Williams, J.P., Weiser, M.R., Pechet, T.T.V., Kobzik, L., Moore, F.D., Hechtman, H.B.: α1-Acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. 273(5), (1997)
van Furth, R., Kramps, J.A., Diesselhof-den Dulk, M.M.: Synthesis of alpha 1-anti-trypsin by human monocytes. Clin. Exp. Immunol. 51(3), 551–557 (1983)
Perlmutter, D.H., Kay, R.M., Cole, F.S., Rossing, T.H., Van Thiel, D., Colten, H.R.: The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc. Natl. Acad. Sci. 82(20), 6918–6921 (1985)
Perlmutter, D.H., Daniels, J.D., Auerbach, H.S., De Schryver-Kecskemeti, K., Winter, H.S., Alpers, D.H.: The alpha 1-antitrypsin gene is expressed in a human intestinal epithelial cell line. J. Biol. Chem. 264(16), 9485–9490 (1989)
Twining, S.S., Fukuchi, T., Yue, B.Y., Wilson, P.M., Boskovic, G.: Corneal synthesis of alpha 1-proteinase inhibitor (alpha 1-antitrypsin). Invest. Ophthalmol. Vis. Sci. 35(2), 458–462 (1994)
Haab, B.B., Geierstanger, B.H., Michailidis, G., Vitzthum, F., Forrester, S., Okon, R., Saviranta, P., Brinker, A., Sorette, M., Perlee, L., Suresh, S., Drwal, G., Adkins, J.N., Omenn, G.S.: Immunoassay and antibody microarray analysis of the HUPO plasma proteome project reference specimens: systematic variation between sample types and calibration of mass spectrometry data. Proteomics 5(13), 3278–3291 (2005)
Kalsheker, N., Morley, S., Morgan, K.: Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochem. Soc. Trans. 30(2), 93–98 (2002)
Perlmutter, D.H.: Liver injury in alpha1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J. Clin. Invest. 110(11), 1579–1583 (2002)
Boskovic, G., Twining, S.S.: Retinol and retinaldehyde specifically increase alpha1-proteinase inhibitor in the human cornea. Biochem. J. 322(Pt 3), 751–756 (1997)
Beatty, K., Bieth, J., Travis, J.: Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J. Biol. Chem. 255(9), 3931–3934 (1980)
Carrell, R., Travis, J.: αAntitrypsin and the serpins: variation and countervariation. Trends Biochem. Sci. 10(1), 20–24 (1985)
Bergin, D.A., Hurley, K., McElvaney, N.G., Reeves, E.P.: Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch. Immunol. Ther. Exp. 60(2), 81–97 (2012)
Grimstein, C., Choi, Y.-K., Wasserfall, C.H., Satoh, M., Atkinson, M.A., Brantly, M.L., Campbell-Thompson, M., Song, S.: Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model. J. Transl. Med. 9(21), 1–13 (2011)
Mele, M., Ferreira, P.G., Reverter, F., DeLuca, D.S., Monlong, J., Sammeth, M., Young, T.R., Goldmann, J.M., Pervouchine, D.D., Sullivan, T.J., Johnson, R., Segre, A.V., Djebali, S., Niarchou, A., Consortium, G.T., Wright, F.A., Lappalainen, T., Calvo, M., Getz, G., Dermitzakis, E.T., Ardlie, K.G., Guigo, R.: Human genomics. The human transcriptome across tissues and individuals. Science 348(6235), 660–665 (2015)
Udby, L., Sørensen, O.E., Pass, J., Johnsen, A.H., Behrendt, N., Borregaard, N., Kjeldsen, L.: Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma. Biochemistry 43(40), 12877–12886 (2004)
Zeng, Z., Hincapie, M., Haab, B.B., Hanash, S., Pitteri, S.J., Kluck, S., Hogan, J.M., Kennedy, J., Hancock, W.S.: The development of an integrated platform to identify breast cancer glycoproteome changes in human serum. J. Chromatogr. A 1217(19), 3307–3315 (2010)
Yoon, S.Y., Kim, J.M., Oh, J.H., Jeon, Y.J., Lee, D.S., Kim, J.H., Choi, J.Y., Ahn, B.M., Kim, S., Yoo, H.S., Kim, Y.S., Kim, N.S.: Gene expression profiling of human HBV- and/or HCV-associated hepatocellular carcinoma cells using expressed sequence tags. Int. J. Oncol. 29(2), 315–327 (2006)
Tian, M., Cui, Y.Z., Song, G.H., Zong, M.J., Zhou, X.Y., Chen, Y., Han, J.X.: Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer 8, 241 (2008)
Kreunin, P., Zhao, J., Rosser, C., Urquidi, V., Lubman, D.M., Goodison, S.: Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J. Proteome Res. 6(7), 2631–2639 (2007)
Piyaphanee, N., Ma, Q., Kremen, O., Czech, K., Greis, K., Mitsnefes, M., Devarajan, P., Bennett, M.R.: Discovery and initial validation of alpha 1-B glycoprotein fragmentation as a differential urinary biomarker in pediatric steroid-resistant nephrotic syndrome. Proteomics Clin. Appl. 5(5–6), 334–342 (2011)
Biswas, S., Sharma, S., Saroha, A., Bhakuni, D., Malhotra, R., Zahur, M., Oellerich, M., Das, H.R., Asif, A.R.: Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach. PLoS One 8(2), e56246 (2013)
Brown, W.M., Dziegielewska, K.M., Saunders, N.R., Christie, D.L., Nawratil, P., Muller-Esterl, W.: The nucleotide and deduced amino acid structures of sheep and pig fetuin. Common structural features of the mammalian fetuin family. Eur. J. Biochem. 205(1), 321–331 (1992)
Haglund, A.C., Ek, B., Ek, P.: Phosphorylation of human plasma alpha2-Heremans-Schmid glycoprotein (human fetuin) in vivo. Biochem. J. 357(Pt 2), 437–445 (2001)
Brylka, L., Jahnen-Dechent, W.: The role of fetuin-A in physiological and pathological mineralization. Calcif. Tissue Int. 93(4), 355–364 (2013)
Jahnen-Dechent, W., Heiss, A., Schafer, C., Ketteler, M.: Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 108(12), 1494–1509 (2011)
Ray, S., Lukyanov, P., Ochieng, J.: Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim. Biophys. Acta 1652(2), 91–102 (2003)
Mathews, S.T., Chellam, N., Srinivas, P.R., Cintron, V.J., Leon, M.A., Goustin, A.S., Grunberger, G.: Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol. Cell. Endocrinol. 164(1–2), 87–98 (2000)
Ix, J.H., Sharma, K.: Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J. Am. Soc. Nephrol. 21(3), 406–412 (2010)
Wang, H., Zhang, M., Soda, K., Sama, A., Tracey, K.J.: Fetuin protects the fetus from TNF. Lancet 350(9081), 861–862 (1997)
Coan, M.H., Roberts, R.C.: A redetermination of the concentration of alpha 2-macroglobulin in human plasma. Biol. Chem. Hoppe Seyler 370(7), 673–676 (1989)
Gonias, S.L., Pizzo, S.V.: Conformation and protease binding activity of binary and ternary human alpha 2-macroglobulin-protease complexes. J. Biol. Chem. 258(23), 14682–14685 (1983)
Thieme, R., Kurz, S., Kolb, M., Debebe, T., Holtze, S., Morhart, M., Huse, K., Szafranski, K., Platzer, M., Hildebrandt, T.B., Birkenmeier, G.: Analysis of alpha-2 macroglobulin from the long-lived and cancer-resistant naked mole-rat and human plasma. PLoS One 10(6), e0130470 (2015)
Borth, W.: Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 6(15), 3345–3353 (1992)
Bock, S.C., Wion, L.L., Vehar, G.A., Lawn, R.M.: Cloning and expression of the cDNA for human antithrombin III. Nucleic Acids Res. 10(24), 8113–8125 (1982)
Koide, T.: Isolation and characterization of antithrombin III from human, porcine and rabbit plasma, and rat serum. J. Biochem. 86(6), 1841–1850 (1979)
Chandra, T., Stackhouse, R., Kidd, V.J., Woo, S.L.: Isolation and sequence characterization of a cDNA clone of human antithrombin III. Proc. Natl. Acad. Sci. U. S. A. 80(7), 1845–1848 (1983)
Conard, J., Brosstad, F., Lie Larsen, M., Samama, M., Abildgaard, U.: Molar antithrombin concentration in normal human plasma. Haemostasis 13(6), 363–368 (1983)
Maclean, P.S., Tait, R.C.: Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs 67(10), 1429–1440 (2007)
Rosenberg, R.D., Damus, P.S.: The purification and mechanism of action of human antithrombin-heparin cofactor. J. Biol. Chem. 248(18), 6490–6505 (1973)
Rosenberg, J.S., McKenna, P.W., Rosenberg, R.D.: Inhibition of human factor IXa by human antithrombin. J. Biol. Chem. 250(23), 8883–8888 (1975)
Patnaik, M.M., Moll, S.: Inherited antithrombin deficiency: a review. Haemophilia Off. J. World Fed. Hemophilia 14(6), 1229–1239 (2008)
Johs, A., Hammel, M., Waldner, I., May, R.P., Laggner, P., Prassl, R.: Modular structure of solubilized human apolipoprotein B-100. Low resolution model revealed by small angle neutron scattering. J. Biol. Chem. 281(28), 19732–19739 (2006)
Yang, C.Y., Gu, Z.W., Weng, S.A., Kim, T.W., Chen, S.H., Pownall, H.J., Sharp, P.M., Liu, S.W., Li, W.H., Gotto, A.M., Chan, L.: Structure of apolipoprotein B-100 of human low-density lipoproteins. Arteriosclerosis 9(1), 96–108 (1989)
Chen, S.-H., Habib, G., Yang, C.-Y., Gu, Z.-W., Lee, B.R., Weng, S.-A., Cai, S., Deslypere, J., Rosseneu, M.: Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science 238(4825), 363–366 (1987)
Young, S.G., Bertics, S.J., Scott, T.M., Dubois, B.W., Curtiss, L.K., Witztum, J.L.: Parallel expression of the MB19 genetic polymorphism in apoprotein B-100 and apoprotein B-48. Evidence that both apoproteins are products of the same gene. J. Biol. Chem. 261(7), 2995–2998 (1986)
Levy, E., Marcel, Y., Deckelbaum, R.J., Milne, R., Lepage, G., Seidman, E., Bendayan, M., Roy, C.C.: Intestinal apoB synthesis, lipids, and lipoproteins in chylomicron retention disease. J. Lipid Res. 28(11), 1263–1274 (1987)
McQueen, M.J., Hawken, S., Wang, X., Ounpuu, S., Sniderman, A., Probstfield, J., Steyn, K., Sanderson, J.E., Hasani, M., Volkova, E., Kazmi, K., Yusuf, S.: Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case–control study. Lancet 372(9634), 224–233 (2008)
Cnop, M., Havel, P., Utzschneider, K., Carr, D., Sinha, M., Boyko, E., Retzlaff, B., Knopp, R., Brunzell, J., Kahn, S.E.: Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46(4), 459–469 (2003)
Olofsson, S.O., Boren, J.: Apolipoprotein B secretory regulation by degradation. Arterioscler. Thromb. Vasc. Biol. 32(6), 1334–1338 (2012)
Olofsson, S.O., Bjursell, G., Bostrom, K., Carlsson, P., Elovson, J., Protter, A.A., Reuben, M.A., Bondjers, G.: Apolipoprotein-B – structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis 68(1–2), 1–17 (1987)
Vukmirica, J., Nishimaki-Mogami, T., Tran, K., Shan, J., McLeod, R.S., Yuan, J., Yao, Z.: The N-linked oligosaccharides at the amino terminus of human apoB are important for the assembly and secretion of VLDL. J. Lipid Res. 43(9), 1496–1507 (2002)
Garner, B., Merry, A.H., Royle, L., Harvey, D.J., Rudd, P.M., Thillet, J.: Structural elucidation of the N-andO-glycans of human apolipoprotein (a) role of O-glycans in conferring protease resistance. J. Biol. Chem. 276(25), 22200–22208 (2001)
Tarugi, P., Lonardo, A., Gabelli, C., Sala, F., Ballarini, G., Cortella, I., Previato, L., Bertolini, S., Cordera, R., Calandra, S.: Phenotypic expression of familial hypobetalipoproteinemia in three kindreds with mutations of apolipoprotein B gene. J. Lipid Res. 42(10), 1552–1561 (2001)
Collins, D.R., Knott, T.J., Pease, R.J., Powell, L.M., Wallis, S.C., Robertson, S., Pullinger, C.R., Milne, R.W., Marcel, Y.L., Humphries, S.E.: Truncated variants of apolipoprotein B cause hypobetalipoproteinaemia. Nucleic Acids Res. 16(17), 8361–8375 (1988)
Raal, F.J., Santos, R.D., Blom, D.J., Marais, A.D., Charng, M.-J., Cromwell, W.C., Lachmann, R.H., Gaudet, D., Tan, J.L., Chasan-Taber, S., Tribble, D.L., Flaim, J.D., Crooke, S.T.: Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375(9719), 998–1006 (2010)
Yang, C.Y., Gu, Z.W., Blancovaca, F., Gaskell, S.J., Yang, M.L., Massey, J.B., Gotto, A.M., Pownall, H.J.: Structure of human apolipoprotein-D - locations of the intermolecular and intramolecular disulfide links. Biochemistry 33(41), 12451–12455 (1994)
Blanco-Vaca, F., Via, D.P., Yang, C.Y., Massey, J.B., Pownall, H.J.: Characterization of disulfide-linked heterodimers containing apolipoprotein D in human plasma lipoproteins. J. Lipid Res. 33(12), 1785–1796 (1992)
Drayna, D.T., Mclean, J.W., Wion, K.L., Trent, J.M., Drabkin, H.A., Lawn, R.M.: Human apolipoprotein-D gene - gene sequence, chromosome localization, and homology to the alpha-2u-globulin superfamily. DNA-J. Mol. Cell Biol. 6(3), 199–204 (1987)
Bajo-Graneras, R., Crespo-Sanjuan, J., Garcia-Centeno, R.M., Garrote-Adrados, J.A., Gutierrez, G., Garcia-Tejeiro, M., Aguirre-Gervas, B., Calvo-Nieves, M.D., Bustamante, R., Ganfornina, M.D., Sanchez, D.: Expression and potential role of apolipoprotein D on the death-survival balance of human colorectal cancer cells under oxidative stress conditions. Int. J. Color. Dis. 28(6), 751–766 (2013)
Curry, M.D., Mcconathy, W.J., Alaupovic, P.: Quantitative-determination of human apolipoprotein-D by electroimmunoassay and radial immunodiffusion. Biochim. Biophys. Acta 491(1), 232–241 (1977)
Camato, R., Marcel, Y.L., Milne, R.W., Lussiercacan, S., Weech, P.K.: Protein polymorphism of a human-plasma apolipoprotein-D antigenic epitope. J. Lipid Res. 30(6), 865–875 (1989)
Kostner, G.: Studies on the cofactor requirements for lecithin: cholesterol acyltransferase. Scand. J. Clin. Lab. Invest. 33(S137), 19–21 (1974)
Perdomo, G., Henry Dong, H.: Apolipoprotein D in lipid metabolism and its functional implication in atherosclerosis and aging. Aging 1(1), 17–27 (2009)
Rassart, E., Bedirian, A., Do Carmo, S., Guinard, O., Sirois, J., Terrisse, L., Milne, R.: Apolipoprotein D. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1482(1–2), 185–198 (2000)
Balbin, M., Freije, J.M.P., Fueyo, A., Sanchez, L.M., Lopezotin, C.: Apolipoprotein-D is the major protein-component in cyst fluid from women with human breast gross cystic-disease. Biochem. J. 271(3), 803–807 (1990)
Kesner, L., Yu, W., BRADLOW, H.: Cyst fluid proteases. Ann. N. Y. Acad. Sci. 586(1), 198–203 (1990)
Kesner, L., Yu, W., Bradlow, H.L., Breed, C.W., Fleisher, M.: Proteases in cyst fluid from human gross cyst breast disease. Cancer Res. 48(22), 6379–6383 (1988)
Boyles, J.K., Notterpek, L.M., Anderson, L.J.: Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J. Biol. Chem. 265(29), 17805–17815 (1990)
Belloir, B., Kovari, E., Surini-Demiri, M., Savioz, A.: Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J. Neurosci. Res. 64(1), 61–69 (2001)
Navarro, A., del Valle, E., Juarez, A., Martinez, E., Ordonez, C., Astudillo, A., Tolivia, J.: Apolipoprotein D synthesis progressively increases in frontal cortex during human lifespan. Age 32(1), 85–96 (2010)
Ordonez, C., Navarro, A., Perez, C., Martinez, E., del Valle, E., Tolivia, J.: Gender differences in apolipoprotein D expression during aging and in Alzheimer disease. Neurobiol. Aging 33(2), 433 e411–433 e420 (2012)
Day, J.R., Albers, J.J., Gilbert, T.L., Whitmore, T.E., McConathy, W.J., Wolfbauer, G.: Purification and molecular-cloning of human apolipoprotein F. Biochem. Biophys. Res. Commun. 203(2), 1146–1151 (1994)
Wang, X., Driscoll, D.M., Morton, R.E.: Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J. Biol. Chem. 274(3), 1814–1820 (1999)
Morton, R.E., Greene, D.J.: Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J. Lipid Res. 35(5), 836–847 (1994)
Reid, K.B., Day, A.J.: Structure-function relationships of the complement components. Immunol. Today 10(6), 177–180 (1989)
Polz, E., Kostner, G.M.: The binding of beta 2-glycoprotein-I to human serum lipoproteins: distribution among density fractions. FEBS Lett 102(1), 183–186 (1979)
Kroll, J., Larsen, J.K., Loft, H., Ezban, M., Wallevik, K., Faber, M.: DNA-binding proteins in Yoshida ascites tumor fluid. Biochim. Biophys. Acta 434(2), 490–501 (1976)
Kochl, S., Fresser, F., Lobentanz, E., Baier, G., Utermann, G.: Novel interaction of apolipoprotein(a) with beta-2 glycoprotein I mediated by the kringle IV domain. Blood 90(4), 1482–1489 (1997)
Kuwana, M., Matsuura, E., Kobayashi, K., Okazaki, Y., Kaburaki, J., Ikeda, Y., Kawakami, Y.: Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood 105(4), 1552–1557 (2005)
Hammel, M., Kriechbaum, M., Gries, A., Kostner, G.M., Laggner, P., Prassl, R.: Solution structure of human and bovine beta(2)-glycoprotein I revealed by small-angle X-ray scattering. J. Mol. Biol. 321(1), 85–97 (2002)
de Laat, B., de Groot, P.G., Derksen, R.H., Urbanus, R.T., Mertens, K., Rosendaal, F.R., Doggen, C.J.: Association between beta2-glycoprotein I plasma levels and the risk of myocardial infarction in older men. Blood 114(17), 3656–3661 (2009)
Twomey, P.J., Viljoen, A., House, I.M., Reynolds, T.M., Wierzbicki, A.S.: Relationship between serum copper, ceruloplasmin, and non–ceruloplasmin-bound copper in routine clinical practice. Clin. Chem. 51(8), 1558–1559 (2005)
Mackiewicz, A., Ganapathi, M., Schultz, D., Kushner, I.: Monokines regulate glycosylation of acute-phase proteins. J. Exp. Med. 166(1), 253–258 (1987)
Herrick, S., Blanc-Brude, O., Gray, A., Laurent, G.: Fibrinogen. Int. J. Biochem. Cell Biol. 31(7), 741–746 (1999)
Doolittle, R.F.: Fibrinogen and fibrin. Annu. Rev. Biochem. 53(1), 195–229 (1984)
Kollman, J.M., Pandi, L., Sawaya, M.R., Riley, M., Doolittle, R.F.: Crystal structure of human fibrinogen. Biochemistry 48(18), 3877–3886 (2009)
Fuss, C., Palmaz, J.C., Sprague, E.A.: Fibrinogen: structure, function, and surface interactions. J. Vasc. Interv. Radiol. 12(6), 677–682 (2001)
Pieters, M., de Maat, M.P.M., Jerling, J.C., Hoekstra, T., Kruger, A.: Fibrinogen concentration and its role in CVD risk in black South Africans – effect of urbanisation. Thromb. Haemost. 106(9), 448–456 (2011)
Carling, M.S., Jeppsson, A., Wessberg, P., Henriksson, A., Baghaei, F., Brisby, H.: Preoperative fibrinogen plasma concentration is associated with perioperative bleeding and transfusion requirements in scoliosis surgery. Spine 36(7), 549–555 (2011). doi:10.1097/BRS.1090b1013e3181d1952dc
Lang, T., Johanning, K., Metzler, H., Piepenbrock, S., Solomon, C., Rahe-Meyer, N., Tanaka, K.A.: The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth. Analg. 108(3), 751–758 (2009)
Henschen, A., Mcdonagh, J.: Fibrinogen, fibrin and factor XIII. New Compr. Biochem. 13, 171–241 (1986)
Zaidi, T., McIntire, L., Farrell, D., Thiagarajan, P.: Adhesion of platelets to surface-bound fibrinogen under flow. Blood 88(8), 2967–2972 (1996)
Clark, R.A., Lanigan, J.M., DellaPelle, P., Manseau, E., Dvorak, H.F., Colvin, R.B.: Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J. Investig. Dermatol. 79(5), 264–269 (1982)
Hogg, D.H., Blombäck, B.: The mechanism of the fibrinogen-thrombin reaction. Thromb. Res. 12(6), 953–964 (1978)
Johnson, L.L., Berggren, K.N., Szaba, F.M., Chen, W., Smiley, S.T.: Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197(6), 801–806 (2003)
Flick, M.J., Du, X., Degen, J.L.: Fibrin (ogen)-αMβ2 interactions regulate leukocyte function and innate immunity in vivo. Exp. Biol. Med. 229(11), 1105–1110 (2004)
Kaptoge, S., White, I., Thompson, S., Wood, A., Lewington, S., Lowe, G., Danesh, J.: Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am. J. Epidemiol. 166(8), 867–879 (2007)
Li, P., Gao, X.H., Chen, H.D., Zhang, Y., Wang, Y., Wang, H., Wang, Y., Xie, Y.: Localization of haptoglobin in normal human skin and some skin diseases. Int. J. Dermatol. 44(4), 280–284 (2005)
Smeets, M.B., Fontijn, J., Kavelaars, A., Pasterkamp, G., De Kleijn, D.P.V.: The acute phase protein haptoglobin is locally expressed in arthritic and oncological tissues. Int. J. Exp. Pathol. 84(2), 69–74 (2003)
Raugei, G., Bensi, G., Colantuoni, V., Romano, V., Santoro, C., Costanzo, F., Cortese, R.: Sequence of human haptoglobin cDNA: evidence that the alpha and beta subunits are coded by the same mRNA. Nucleic Acids Res. 11(17), 5811–5819 (1983)
Dobryszycka, W.: Biological functions of haptoglobin - new pieces to an old puzzle. Eur. J. Clin. Chem. Clin. Biochem. 35(9), 647–654 (1997)
Galicia, G., Ceuppens, J.L.: Haptoglobin Function and Regulation in Autoimmune Diseases. INTECH Open Access Publisher, (2011)
Van Vlierberghe, H., Langlois, M., Delanghe, J.: Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin. Chim. Acta 345(1–2), 35–42 (2004)
Carter, K., Worwood, M.: Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int. J. Lab. Hematol. 29(2), 92–110 (2007)
Moretti, J., Borel, J., Dobryszycka, W., Jayle, M.-F.: Détermination de la demi-vie de l’haptoglobine plasmatique humaine. Biochim. Biophys. Acta 69, 205–211 (1963)
Bharti, A., Ma, P.C., Maulik, G., Singh, R., Khan, E., Skarin, A.T., Salgia, R.: Haptoglobin alpha-subunit and hepatocyte growth factor can potentially serve as serum tumor biomarkers in small cell lung cancer. Anticancer Res. 24(2C), 1031–1038 (2004)
Lim, Y.K., Jenner, A., Ali, A.B., Wang, Y., Hsu, S.I., Chong, S.M., Baumman, H., Halliwell, B., Lim, S.K.: Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 58(3), 1033–1044 (2000)
Tseng, C.F., Lin, C.C., Huang, H.Y., Liu, H.C., Mao, S.J.T.: Antioxidant role of human haptoglobin. Proteomics 4(8), 2221–2228 (2004)
Delanghe, J.R., Langlois, M.R.: Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin. Chim. Acta 312(1–2), 13–23 (2001)
Tolosano, E., Altruda, F.: Hemopexin: structure, function, and regulation. DNA Cell Biol. 21(4), 297–306 (2002)
Takahashi, N., Takahashi, Y., Putnam, F.W.: Complete amino acid sequence of human hemopexin, the heme-binding protein of serum. Proc. Natl. Acad. Sci. U. S. A. 82(1), 73–77 (1985)
Weeke, B., Krasilnikoff, P.A.: The concentration of 21 serum proteins in normal children and adults. Acta Med. Scand. 192(1–6), 149–155 (1972)
Kanakoudi, F., Drossou, V., Tzimouli, V., Diamanti, E., Konstantinidis, T., Germenis, A., Kremenopoulos, G.: Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin. Chem. 41(4), 605–608 (1995)
Poon, I.K., Patel, K.K., Davis, D.S., Parish, C.R., Hulett, M.D.: Histidine-rich glycoprotein: the Swiss Army knife of mammalian plasma. Blood 117(7), 2093–2101 (2011)
Kassaar, O., McMahon, S.A., Thompson, R., Botting, C.H., Naismith, J.H., Stewart, A.J.: Crystal structure of histidine-rich glycoprotein N2 domain reveals redox activity at an interdomain disulfide bridge: implications for angiogenic regulation. Blood 123(12), 1948–1955 (2014)
Borza, D.B., Tatum, F.M., Morgan, W.T.: Domain structure and conformation of histidine-proline-rich glycoprotein. Biochemistry 35(6), 1925–1934 (1996)
Saito, H., Goodnough, L.T., Boyle, J.M., Heimburger, N.: Reduced histidine-rich glycoprotein levels in plasma of patients with advanced liver cirrhosis. Possible implications for enhanced fibrinolysis. Am. J. Med. 73(2), 179–182 (1982)
Drasin, T., Sahud, M.: Blood-type and age affect human plasma levels of histidine-rich glycoprotein in a large population. Thromb. Res. 84(3), 179–188 (1996)
Leung, L.L., Harpel, P.C., Nachman, R.L., Rabellino, E.M.: Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood 62(5), 1016–1021 (1983)
Corrigan Jr., J.J., Jeter, M.A., Bruck, D., Feinberg, W.M.: Histidine-rich glycoprotein levels in children: the effect of age. Thromb. Res. 59(3), 681–686 (1990)
Jones, A.L., Hulett, M.D., Parish, C.R.: Histidine-rich glycoprotein: a novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell Biol. 83(2), 106–118 (2005)
Saigo, K., Yoshida, A., Ryo, R., Yamaguchi, N., Leung, L.L.: Histidine-rich glycoprotein as a negative acute phase reactant. Am. J. Hematol. 34(2), 149–150 (1990)
Griffin, J.H., Cochrane, C.G.: Mechanisms for the involvement of high molecular weight kininogen in surface-dependent reactions of Hageman factor. Proc. Natl. Acad. Sci. 73(8), 2554–2558 (1976)
Kaplan, A.P., Silverberg, M.: The coagulation-kinin pathway of human plasma. Blood 70(1), 1–15 (1987)
Margolius, H.S.: Kallikreins and kinins: some unanswered questions about system characteristics and roles in human disease. Hypertension 26(2), 221–229 (1995)
Weisel, J.W., Nagaswami, C., Woodhead, J.L., DeLa Cadena, R.A., Page, J.D., Colman, R.W.: The shape of high molecular weight kininogen. Organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J. Biol. Chem. 269(13), 10100–10106 (1994)
Thompson, R.E., Mandle Jr., R., Kaplan, A.P.: Association of factor XI and high molecular weight kininogen in human plasma. J. Clin. Invest. 60(6), 1376 (1977)
Mandle, R.J., Colman, R.W., Kaplan, A.P.: Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc. Natl. Acad. Sci. 73(11), 4179–4183 (1976)
Reddigari, S., Kaplan, A.P.: Quantification of human high molecular weight kininogen by immunoblotting with a monoclonal anti-light chain antibody. J. Immunol. Methods 119(1), 19–25 (1989)
Adam, A., Albert, A., Calay, G., Closset, J., Damas, J., Franchimont, P.: Human kininogens of low and high molecular mass: quantification by radioimmunoassay and determination of reference values. Clin. Chem. 31(3), 423–426 (1985)
Gomme, P.T., McCann, K.B., Bertolini, J.: Transferrin: structure, function and potential therapeutic actions. Drug Discov. Today 10(4), 267–273 (2005)
Bailey, S., Evans, R.W., Garratt, R.C., Gorinsky, B., Hasnain, S., Horsburgh, C., Jhoti, H., Lindley, P.F., Mydin, A.: Molecular structure of serum transferrin at 3.3-.ANG. resolution. Biochemistry 27(15), 5804–5812 (1988)
Kurokawa, H., Mikami, B., Hirose, M.: Crystal structure of diferric hen ovotransferrin at 2.4 Å resolution. J. Mol. Biol. 254(2), 196–207 (1995)
Vahlquist, A., Rask, L., Peterson, P.A., Berg, T.: The concentrations of retinol-binding protein, prealbumin, and transferrin in the sera of newly delivered mothers and children of various ages. Scand. J. Clin. Lab. Invest. 35(6), 569–575 (1975)
Chitambar, C.R., Matthaeus, W.G., Antholine, W.E., Graff, K., O’Brien, W.J.: Inhibition of leukemic HL60 cell growth by transferrin-gallium: effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood 72(6), 1930–1936 (1988)
Trowbridge, I.S., Shackelford, D.A.: Structure and function of transferrin receptors and their relationship to cell-growth. Biochem. Soc. Symp. 51, 117–129 (1986)
Koterov, A.N., Pushkareva, N.B., Nikol’skii, A.V.: The radiation-modifying capacity of xenogenic apotransferrin for the number of endogenous colony forming units in the spleen of irradiated mice. Radiats. Biol. Radioecol. Ross. Akad. Nauk 43(6), 647–653 (2003)
Daniels, T.R., Delgado, T., Helguera, G., Penichet, M.L.: The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 121(2), 159–176 (2006)
Daniels, T.R., Delgado, T., Rodriguez, J.A., Helguera, G., Penichet, M.L.: The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 121(2), 144–158 (2006)
Preissner, K.T.: Structure and biological role of vitronectin. Annu. Rev. Cell Biol. 7(1), 275–310 (1991)
Preissner, K.T., Reuning, U.: Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin. Thromb. Hemost. 37(04), 408–424 (2011)
Preissner, K.T., Seiffert, D.: Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb. Res. 89(1), 1–21 (1998)
Schvartz, I., Seger, D., Shaltiel, S.: Vitronectin. Int. J. Biochem. Cell Biol. 31(5), 539–544 (1999)
Montaldo, C., Mattei, S., Baiocchini, A., Rotiroti, N., Nonno, F.D., Pucillo, L.P., Cozzolino, A.M., Battistelli, C., Amicone, L., Ippolito, G., van Noort, V., Conigliaro, A., Alonzi, T., Tripodi, M., Mancone, C.: Spike-in SILAC proteomic approach reveals the vitronectin as an early molecular signature of liver fibrosis in hepatitis C infections with hepatic iron overload. Proteomics 14(9), 1107–1115 (2014)
Seiffert, D., Geisterfer, M., Gauldie, J., Young, E., Podor, T.J.: IL-6 stimulates vitronectin gene expression in vivo. J. Immunol. 155(6), 3180–3185 (1995)
Chauhan, A.K., Moore, T.L.: Presence of plasma complement regulatory proteins clusterin (Apo J) and vitronectin (S40) on circulating immune complexes (CIC). Clin. Exp. Immunol. 145(3), 398–406 (2006)
Tsai, J.S., Chen, S.C., Huang, K.C., Lue, B.H., Lee, L.T., Chiu, T.Y., Chen, C.Y., Guo, F.R., Chuang, L.M.: Plasma zinc alpha2-glycoprotein levels are elevated in smokers and correlated with metabolic syndrome. Eur. J. Clin. Investig. 45(5), 452–459 (2015)
Burgi, W., Schmid, K.: Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J. Biol. Chem. 236, 1066–1074 (1961)
Hassan, M.I., Waheed, A., Yadav, S., Singh, T.P., Ahmad, F.: Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol. Cancer Res. 6(6), 892–906 (2008)
van Egmond, M., Damen, C.A., van Spriel, A.B., Vidarsson, G., van Garderen, E., van de Winkel, J.G.: IgA and the IgA Fc receptor. Trends Immunol. 22(4), 205–211 (2001)
Woof, J.M., Kerr, M.A.: The function of immunoglobulin A in immunity. J. Pathol. 208(2), 270–282 (2006)
Kutteh, W., Prince, S., Mestecky, J.: Tissue origins of human polymeric and monomeric IgA. J. Immunol. 128(2), 990–995 (1982)
Rowe, D.S., Anderson, S.G., Tackett, L.: A research standard for human serum immunoglobulin D. Bull. World Health Organ. 43(4), 607–609 (1970)
Rowe, D.S., Fahey, J.L.: A new class of human immunoglobulins. Ii. Normal Serum Igd. J Exp Med 121, 185–199 (1965)
Zegers, B.J., Stoop, J.W., Reerink-Brongers, E.E., Sander, P.C., Aalberse, R.C., Ballieux, R.E.: Serum immunoglobulins in healthy children and adults. Levels of the five classes, expressed in international units per millilitre. Clin. Chim. Acta 65(3), 319–329 (1975)
Rogentine, G.N., Rowe, D.S., Bradley, J., Waldmann, T.A., Fahey, J.L.: Metabolism of human immunoglobulin D (IgD). J. Clin. Invest. 45(9), 1467–1478 (1966)
Van Boxel, J.A., Paul, W.E., Terry, W.D., Green, I.: Communications. IgD-bearing human lymphocytes. J. Immunol. 109(3), 648–651 (1972)
Murphy, K.M.: Janeway’s Immunobiology. Garland Science, (2011)
Waxdal, M.J., Konigsberg, W.H., Henley, W.L., Edelman, G.M.: The covalent structure of a human G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry 7(5), 1959–1966 (1968)
Johansson, S.G.: Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet 2(7523), 951–953 (1967)
Gounni, A.S., Lamkhioued, B., Ochiai, K., Tanaka, Y., Delaporte, E., Capron, A., Kinet, J.P., Capron, M.: High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 367(6459), 183–186 (1994)
Gould, H.J., Sutton, B.J.: IgE in allergy and asthma today. Nat. Rev. Immunol. 8(3), 205–217 (2008)
Spiegelberg, H.L., Fishkin, B.G.: The catabolism of human γG immunoglobulins of different heavy chain subclasses. III. The catabolism of heavy chain disease proteins and of Fc fragments of myeloma proteins. Clin. Exp. Immunol. 10(4), 599–607 (1972)
Spiegelberg, H.L., Fishkin, B.G., Grey, H.M.: Catabolism of human gammaG-immunoglobulins of different heavy chain subclasses. I. Catabolism of gammaG-myeloma proteins in man. J. Clin. Invest. 47(10), 2323–2330 (1968)
Jefferis, R.: Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8(3), 226–234 (2009)
Balbin, M., Grubb, A., de Lange, G.G., Grubb, R.: DNA sequences specific for Caucasian G3m(b) and (g) allotypes: allotyping at the genomic level. Immunogenetics 39(3), 187–193 (1994)
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P.: Molecular Biology of the Cell (4th edition). Garland Science, (2003)
Gonzalez-Quintela, A., Alende, R., Gude, F., Campos, J., Rey, J., Meijide, L.M., Fernandez-Merino, C., Vidal, C.: Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 151(1), 42–50 (2008)
Schauer, U., Stemberg, F., Rieger, C.H., Borte, M., Schubert, S., Riedel, F., Herz, U., Renz, H., Wick, M., Carr-Smith, H.D., Bradwell, A.R., Herzog, W.: IgG subclass concentrations in certified reference material 470 and reference values for children and adults determined with the binding site reagents. Clin. Chem. 49(11), 1924–1929 (2003)
Larsson, A., Palm, M., Hansson, L.-O., Basu, S., Axelsson, O.V.E.: Reference values for α1-acid glycoprotein, α1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet. Gynecol. Scand. 87(10), 1084–1088 (2008)
Simister, N.E.: Placental transport of immunoglobulin G. Vaccine 21(24), 3365–3369 (2003)
Chames, P., Van Regenmortel, M., Weiss, E., Baty, D.: Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 157(2), 220–233 (2009)
Reusch, D., Haberger, M., Selman, M.H., Bulau, P., Deelder, A.M., Wuhrer, M., Engler, N.: High-throughput work flow for IgG Fc-glycosylation analysis of biotechnological samples. Anal. Biochem. 432(2), 82–89 (2013)
Reusch, D., Tejada, M.L.: Fc glycans of therapeutic antibodies as critical quality attributes (CQAs). Glycobiology (2015)
Ehrenstein, M.R., Notley, C.A.: The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol. 10(11), 778–786 (2010)
Thurnheer, M.C., Zuercher, A.W., Cebra, J.J., Bos, N.A.: B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J. Immunol. 170(9), 4564–4571 (2003)
Randall, T.D., Brewer, J.W., Corley, R.B.: Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J. Biol. Chem. 267(25), 18002–18007 (1992)
Czajkowsky, D.M., Shao, Z.: The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc. Natl. Acad. Sci. 106(35), 14960–14965 (2009)
Quartier, P., Potter, P.K., Ehrenstein, M.R., Walport, M.J., Botto, M.: Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 35(1), 252–260 (2005)
Tofik, R., Ekelund, U., Torffvit, O., Sward, P., Rippe, B., Bakoush, O.: Increased urinary IgM excretion in patients with chest pain due to coronary artery disease. BMC Cardiovasc. Disord. 13(1), 72 (2013)
Caidahl, K., Hartford, M., Karlsson, T., Herlitz, J., Pettersson, K., de Faire, U., Frostegård, J.: IgM-phosphorylcholine autoantibodies and outcome in acute coronary syndromes. Int. J. Cardiol. 167(2), 464–469 (2013)
Arnold, J.N., Saldova, R., Hamid, U.M.A., Rudd, P.M.: Evaluation of the serum n‐linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 8(16), 3284–3293 (2008)
Jaeken, J., Hennet, T., Freeze, H., Matthijs, G.: On the nomenclature of congenital disorders of glycosylation (CDG). J. Inherit. Metab. Dis. 31(6), 669–672 (2008)
Schubert, M., Walczak, M.J., Aebi, M., Wider, G.: Posttranslational modifications of intact proteins detected by NMR spectroscopy: application to glycosylation. Angew. Chem. 127(24), 7202–7206 (2015)
Shubhakar, A., Reiding, K., Gardner, R., Spencer, D.R., Fernandes, D., Wuhrer, M.: High-throughput analysis and automation for glycomics studies. Chromatographia, 1–13 (2014)
Stockmann, H., O’Flaherty, R., Adamczyk, B., Saldova, R., Rudd, P.M.: Automated, high-throughput serum glycoprofiling platform. Integr. Biol. (2015)
Huffman, J.E., Pučić-Baković, M., Klarić, L., Hennig, R., Selman, M.H., Vučković, F., Novokmet, M., Krištić, J., Borowiak, M., Muth, T.: Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol. Cell. Proteomics 13(6), 1598–1610 (2014)
Morell, A.G., Gregoriadis, G., Scheinberg, I.H., Hickman, J., Ashwell, G.: The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 246(5), 1461–1467 (1971)
Acknowledgments
This work was supported by the European Union Seventh Framework Programme projects HighGlycan (Grant No. 278535) and IBD-BIOM (Grant No. 305479) as well as by the Dutch Arthritis Foundation (RF 13-3-201) and a Zenith grant from The Netherlands Organization for Scientific Research (#93511033). We thank Rosina Plomp for her help with reviewing the IgE section.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLSX 38 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Clerc, F., Reiding, K.R., Jansen, B.C. et al. Human plasma protein N-glycosylation. Glycoconj J 33, 309–343 (2016). https://doi.org/10.1007/s10719-015-9626-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-015-9626-2