Abstract
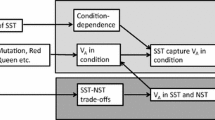
Female preferences for elaborate male sexual traits have been documented in a number of species in which males contribute only genes to the next generation. In such systems, mate choice has been hypothesised to benefit females genetically. For the genetic benefits to be possible there must be additive genetic variation (VA) for sexual ornaments, such that highly ornamented males can pass fitter genes on to the progeny of choosy females. Here, I review the mechanisms that can contribute to the maintenance of this variation. The variation may be limited to sexual ornaments, resulting in Fisherian benefits in terms of the increased reproductive success of male progeny produced by choosy females. Alternatively, ornaments may capture VA in other life-history traits. In the latter case, “good genes” benefits may apply in terms of improved performance of the progeny of either sex. Some mechanisms, however, such as negative pleiotropy, sexually antagonistic variation or overdominance, can maintain VA in ornaments and other life-history traits with little variation in total fitness, leaving little room for any genetic benefits of mate choice. Distinguishing between these mechanisms has consequences not only for the theory of sexual selection, but also for evolution of sex and for biological conservation. I discuss how the traditional ways of testing for genetic benefits can usefully be supplemented by tests detecting benefits resulting from specific mechanisms maintaining VA in sexual ornaments.
Similar content being viewed by others
References
Acevedo-Whitehouse K, Cunningham AA (2006) Is MHC enough for understanding wildlife immunogenetics? Trends Ecol Evol 21:433–438
Agrawal AF (2001) Sexual selection and the maintenance of sexual reproduction. Nature 411:692–695
Alonzo SH, Sinervo B (2001) Mate choice games, context-dependent good genes, and genetic cycles in the sideblotched lizard, Uta stansburiana. Behav Ecol Sociobio 49:176–186
Andersson M (1986) Evolution of condition dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40:804–816
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Aparicio JM, Cordero PJ, Veiga JP (2001) A test of the hypothesis of mate choice based on heterozygosity in the spotless starling. Anim Behav 62:1001–1006
Barber I, Arnott SA (2000) Split-clutch IVF: a technique to examine indirect fitness consequences of mate preferences in sticklebacks. Behaviour 137:1129–1140
Barber I, Arnott SA, Braithwaite VA, Andrew J, Huntingford FA (2001) Indirect fitness consequences of mate choice in sticklebacks:offspring of brighter males grow slowly but resist parasitic infections. Proc R Soc Lond B 268:71–76
Barlow GW (2005) How do we decide that a species is sex-role reversed? Q Rev Biol 80:28–35
Barton NH, Keightley PD (2002) Understanding quantitative genetic variation. Nat Rev Genet 3:11–21
Bartosch-Härlid A, Berlin S, Smith NGC, Moller AP, Ellegren H (2003) Life history and the male mutation bias. Evolution 57:2398–2406
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Blows MW, Brooks R, Kraft PG (2003) Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution 57:1622–1630
Blows MW, Chenoweth SF, Hine E (2004) Orientation of the genetic variance-covariance matrix and the fitness surface for multiple male sexually selected traits. Am Nat 163:329–340
Blows MW, Hoffmann AA (2005) A reassessment of genetic limits to evolutionary change. Ecology 86:1371–1384
Bochdanovits Z, de Jong G (2004) Antagonistic pleiotropy for life-history traits at the gene expression level. Proc R Soc Lond B (Suppl.) 271:S75–S78
Borghans JAM, Beltman JB, De Boer RJ (2004) MHC polymorphism under host-pathogen coevolution. Immunogenetics 55:732–759
Borgia G (1979) Sexual selection and the evolution of mating systems. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in the insects. Academic Press, New York, pp 19–80
Bouck A, Vision T (2007) The molecular ecologist’s guide to expressed sequence tags. Mol Ecol. 16:907–924
Brooks R (2000) Negative genetic correlation between male sexual attractiveness and survival. Nature 406:67–70
Brooks R, Endler JA (2001) Direct and indirect selection and quantitative genetics of male traits in guppies (Poecilla reticulata). Evolution 55:1002–1015
Brown JL (1996) A theory of mate choice based on heterozygosity. Behav Ecol 8:60–65
Brussière LF, Hunt J, Stölting KN, Jennions M, Brooks R (2007) Mate choice for genetic quality when environments vary: suggestions for empirical progress. Genetica (in press)
Byers DL (2005) Evolution in heterogeneous environments and the potential of maintenance of genetic variation in traits of adaptive significance. Genetica 123:107–124
Calsbeek R, Sinervo B (2004) Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J Evol Biol 17:464–470
Castro I, Mason KM, Armstrong DP, Lambert DM (2004) Effect of extra-pair paternity on effective population size in a reintroduced population of the endangered hihi, and potential for behavioural management. Conserv Genet 5:381–393
Charlesworth B, Charlesworth D (1999) The genetic basis of inbreeding depresion. Genet Res 74:329–340
Charlesworth B, Hughes KA (1999) The maintenance of genetic variation in life-history traits. In: Singh RS, Krimbas CB (eds) Evolutionary genetics: from molecules to morphology. Cambridge University Press, Cambridge, pp 369–392
Chippindale AK, Gibson JR, Rice WR (2001) Negative genetic correlation for adult fitness between sexes reveals onogenetic conflict in Drosophila. Proc Natl Acad Sci USA 98:1671–1675
Cordero C, Eberhard WG (2003) Female choice of sexually antagonistic male adaptations:a critical review of some current research. J Evol Biol 16:1–6
Cotton S, Fowler K, Pomiankowski A (2004) Do sexual ornaments demonstrate heightened conditiondependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B 271:771–783
Cunningham EJA, Russell AF (2000) Egg investment is influenced by male attractiveness in the mallard. Nature 404:74–77
Curtsinger JW, Service PM, Prout T (1994) Anatagonistic pleiotropy, reversal of dominance, and genetic polymorphism. Am Nat 144:210–228
Danielson-Francois AM, Kelly JK, Greenfield MD (2006) Genotype × environment interaction for male attractiveness in an acoustic moth: evidence for plasticity and canalization. J Evol Biol 19:532–542
Darwin Ch (1871) The descent of man and selection in relation to sex. John Murray, London
Day T (2000) Sexual selection and the evolution of costly female preferences: spatial effects. Evolution 54:715–730
de Campos-Lima PO, Gavioli R, Zhang QG, Wallace LE, Dolcetti R, Rowe M, Rickinson AB, Masucci. M.G. (2003) HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+ population . Science 260:98–100
Doherty PF, Sorci G, Royle JA, Hines JE, Nichols JD, Boulinier T (2003) Sexual selection affects local extincion and turnover in bird communities. Proc Natl Acad Sci USA 100:5858–5862
Dolgin ES, Whitlock MC, Agrawal AF (2006) Male Drosophila melanogaster have higher mating success when adapted to their thermal environment. J Evol Biol 19:1894–1900
Drake JB, Charlesworth B, Charlesworth D, Crow JF (1998) Rates of spontaneous mutation. Genetics 148:1667–1686
Drost JB, Lee WR (1995) Biological basis of germline mutation: comparisons of spontaneous mutation rates among Drosophila, mouse and human. Environ Mol Mut 25(Suppl. 26):48–64
Ekblom R, Saether SA, Grahn M, Fiske P, Kalas JA, Hoglund J (2004) Major histocompatibility complex variation and mate choice in a lekking bird, the great snipe (Gallinago media). Mol Ecol 31:3821–3828
Endler JA (1980) Natural selection on color pattern in Poecilia reticulata. Evolution 34:76–91
Eshel I, Volovik I, Sansone E (2000) On Fihser-Zahavi’s handicapped sexy son. Evol Ecol Res 2:509–523
Evans JP, Bisazza A, Pilastro A (2004) Female mating preferences for colourful males in a population of guppies subject to high predation. J Fish Biol. 65:1154–1159
Farr JA (1977) Male rarity or novelty, female choice behavior, and sexual selection in the guppy, Poecilla reticulata Peters (Pisces: Poecillidae). Evolution 31:162–168
Fedorka KM, Mousseau TA (2004) Female mating bias results in conflicting sex-specific offspring fitness. Nature 429:65–67
Fisher RA (1930) The genetical theory of natural selection. Oxford Universtiy Press, Oxford
Fitzpatrick MJ (2004) Pleiotropy and the genomic location of sexually selected genes. Am Nat 163:800–808
Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB (2007) Sexually antagonistic genetic variation for fitness in red deer. Nature 447:1107–1109
Fricke C, Arnqvist G (2007) Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): the role of sexual selection. Evolution 61:440–454
Gavrilets S, Arnqvist G, Friberg U (2001) The evolution of female mate choice by sexual conflict. Proc R Soc Lond B 268:531–539
Gilbert L, Williamson KA, Hazon N, Graves JA (2006) Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proc R Soc Lond B 273:1765–1771
Greenfield MD, Rodriguez RL (2004) Genotype-environment interaction and the reliability of mating signals. Anim Behav 64:1461–1468
Hall M, Lindholm AK, Brooks R (2004) Direct selection on male attractiveness and female preference fails to produce a response. BMC Evol Biol 4:1
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–7
Hansson B, Westerberg L (2002) On the correlation between heterozygosity and fitness in natural populations. Mol Ecol 11:2467–2474
Hedrick PW (1986) Genetic polymorphisms in heterogenous environments: a decade later. Ann Rev Ecol Syst 17:735–766
Hedrick PW (1994) Purging inbreeding depression and the probability of extinction: full-sib mating. Heredity 73:363–372
Hedrick PW (1999) Antagonistic pleiotropy and genetic polymorphism: a perspective. Heredity 82:126–133
Hedrick PW, Kim TJ (2000) Genetics of complex polymorphisms: parasites and the mainenance of the major histocompatibilty complex variation. In: Singh RS, Krimbas CB (eds) Evolutionary genetics: from molecules to morphology. Cambridge University Press, New York, pp 204–233
Hine E, Chenoweth SF, Blows MW (2004) Multivariate quantitative genetics and the lek paradox: genetic variance in male sexually selected traits of Drosophila serrata under field conditions. Evolution 58:2754–2762
Holland B (2002) Sexual selection fails to promote adaptation to a new environment. Evolution 56:721–730
Holland B, Rice WR (1998) Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52:1–7
Houde AE (1992) Sex-linked heritability of a sexually selected character in a natural populatoin of Poecilia reticulata. Heredity 69:229–235
Houle D, Kondrashov AS (2002) Coevolution of costly mate choice and condition-dependent display of good genes. Proc R Soc Lond B 269:97–104
Hughes KA, Du L, Rodd FH, Reznick DN (1999) Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim Behav 59:907–916
Hughes KA, Rodd FH, Reznick DN (2005) Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). J Evol Biol 18:35–45
Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiere LF (2004) High-quality male field crickets invest heavily in sexual display but die young. Nature 432:1024–1027
Hunt J, Bussiere LF, Jennions MD, Brooks R (2004) What is genetic quality? Trends Ecol Evol 19:329–333
Hurst LD, Ellegren H (1998) Sex biases in the mutation rate. Trends Genet 14:446–452
Irwin AJ, Taylor PD (2000) Heterozygous advantage and the evolution of female choice. Evol Ecol Res 2:119–128
Iwasa Y., Pomiankowski A (1995) Continual change in mate preferences. Nature 377:420–422
Jaenike J (2001) Sex chromosome meiotic drive. Ann Rev Ecol Syst 32:25–49
Jia FY, Greenfield MD (1997) When are good genes good? Variable outcomes of female choice in wax moths. Proc R Soc Lond B 264:1057–1063
Johns PM, Wolfenbarger LL, Wilkinson GS (2005) Genetic linkage between a sexually selected trait and X chromosome meiotic drive. Proc R Soc Lond B 272:2097–2103
Karkkainen K, Kuittinen H, van Treuren R, Vogl C, Oikarinen S, Savolainen O (1999) Genetic basis of inbreeding depression in Arabis petrea. Evolution 53:1354–1365
Keller L (1999) Levels of selection in evolution. Princeton University Press, Princeton
Kelly JK, Willis JH (2001) Deleterious mutations and genetic variation for flower size in Mimulus guttatus. Evolution 55:937–942
Kirkpatrick M (1982) Sexual selection and the evolution of female choice. Evolution 36:1–12
Kirkpatrick M, Barton NH (1997) The strength of indirect selection on female mating preferences. Proc Natl Acad Sci USA 94:1282–1286
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nature 350:33–38
Kisdi E (2001) Long-term adaptive diversity in Levene-type models. Evol Ecol Res 3:721–727
Klappert K, Reinhold K (2005) Local adaptation and sexual selection: a reciprocal transfer experiment with the grasshopper Chorthippus biguttulus. Behav Ecol Sociobiol 58:36–43
Kokko H (2001) Fisherian and “good genes” benefits of mate choice: how (not) to distinguish between them. Ecol Lett 4:322–326
Kokko H, Brooks R (2003) Sexy to die for? Sexual selection and the risk of extinction. Ann Zool Fennici 40:207–219
Kokko H, Brooks R, Jennions MD, Morley J (2003) The evolution of mate choice and mating biases. Proc R Soc Lond B 270:653–664
Kokko H, Brooks R, McNamara JM, Houston AI (2002) The sexual selection continuum. Proc R Soc Lond B 269:1331–1340
Kokko H, Heubel K (2007) Condition-dependence, genotype-by-environment interactions and the lek paradox. Genetica. doi:10.1007/s10709-007-9166-1
Kokko H, Jennions M, Brooks R (2006) Unifying and testing models of sexual selection. Ann Rev Ecol Evol Syst 37:43–66
Kokko H, Jennions MD, Houde A (2007) Evolution of frequency-dependent mate choice: keeping up with fashion trends. Proc R Soc Lond B 274:1317–1324
Konior M, Keller L, Radwan J (2005) Effect of inbreeding and heritability of sperm competition success in the bulb mite Rhizoglyphus robini. Heredity 94:577–581
Kotiaho JS, Simmons LW, Hunt J, Tomkins JL (2003) Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am Nat 161:852–859
Kotiaho JS, Simmons LW, Tomkins JL (2001) Towards a resolution of the lek paradox. Nature 410:684–686
Kozielska M, Krzeminska A, Radwan J (2004) Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc R Soc Lond B 271:165–170
Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness (2002) Antler size in red deer: herability and selection but no evolution. Evolution 6:1683–1695
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci USA 78:3721–3725
Lande RL, Wilkinson GS (1999) Models of sex-ratio meiotic drive and sexual selection in stalk-eyes flies. Genet Res 74:245–253
Lehmann L, Keller L, Kokko H (2006) Mate choice evolution, dominance effects and the maintenance of genetic variatoin. J Theor Biol. doi: 10.1016/jtbi.2006.07.033
Lennington S, Coopersmitn CB, Erhart M (1989) Female preference and variability anmong t-haplogypes in wild house mice. Am Nat 143:766–784
Levene H (1953) Genetic equilibrium when more than one ecological niche is available. Am Nat 87:331–333
Lynch M, Blanchard J, Houle D, Kibota T, Schultz S, Vassilieva L, Willis J (1999) Perspective: spontaneous deleterious mutations. Evolution 53:645–663
Lynch M, Latta L, Hicks J, Giorgiani M (1998) Mutation, selection and the maintenance of life-history variation in a natural population. Evolution 53:727–733
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Sunderland
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Maynard-Smith J (1998) Evolutionary genetics. Oxford University Press, Oxford
Maynard-Smith J, Hoekstra R (1980) Polymorphism in a varied environment: how robust are the models? Genet Res 35:45–47
Mead LS, Arnold SJ (2004) Quantitative genetic models of sexual selection. Trends Ecol Evol 19:264–271
Merila J, Kruuk LEB, Sheldon BC (2001) Natural selection on the genetical component of variance in body condition in a wild bird population. J Evol Biol 14:918–929
Milinski M, Bakker TCM (1990) Female sticklebacks use male coloration in mate choice and thus avoid parasitised males. Nature 344:330–333
Mills SC, Alatalo RV, Koskela E, Mappes J, Mappes, T, Oksanen TA (2007) Signal reliability compromised by genotype-by-environment interaction and potential mechanisms for its preservation. Evolution 61:1748–1757
Mitton JB, Schuster WS, Cothran EG, De Fries JC (1993) Correlation between the individual heterozygosity of parents and their offspring. Heredity 71:59–63
Moller AP, Alatalo RV (1999) Good-genes effects in sexual selection. Proc R Soc Lond B 266:85–91
Moller AP, Briskie JV (1995) Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav Ecol Sociobiol 36:357–365
Moore AJ, Moore PJ (2006) The genetics of sexual selection. In: Fox CW, Wolf JB (eds) Evolutionary genetics: concepts and case studied. Oxford University Press, Oxford, pp 339–349
Morrow EH, Pitcher TE (2003) Sexual selection and the risk of extinction in birds. Proc R Soc Lond B 270:1793–1799
Müller G, Ward P (1995) Parasitiesm and heterozygosity influence the secondary sexual characters of the Eropean minnow, Phoxinus phoxinus (L.)(Cyprinidae). Ethology 100:309–319
Neff BD (2004) Increased performance of offspring sired by parasitic males in bluegill sunfish. Behav Ecol 15:327–331
Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA (2006) Frequency-dependent survival in natural guppy populations. Nature 441:633–666
Olsson M, Madsen T, Wapstra E, Silverin B, Ujvari B, Wittzell H (2005) MHC, health, color, and reproductive success in sand lizards. Behav Ecol Sociobiol 58:289–294
Oosterhout van C, Trigg RE, Carvalho GR, Magurran AE, Hauser L (2003) Inbreeding depression and genetic load of sexually selected traits: how the guppy lost its spots. J Evol Biol 18:273–281
Ord TJ, Stuart-Fox D (2006) Ornament evolution in dragon lizards: multiple gains and widespread losses reveal a complex history of evolutionary change. J Evol Biol 19:797–808
Partridge L (1983) Non-random mating and offspring fitness. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 227–255
Petrie M, Roberts G (2007) Sexual selection and the evolution of evolvability. Heredity 98:198–205
Piertney SB, Oliver MK (2006) The evolutionary ecology of the major histocompatibility complex. Heredity 96:7–21
Pomiankowski A, Iwasa Y (1998) Runaway ornament diversity caused by Fisherian sexual selection. Proc. Natl. Acad. Sci. USA 95:5106–5111
Pomiankowski A, Iwasa Y, Nee S (1991) The evolution of costly mate preferences. 1. Fisher and biased mutation. Evolution 45:1422–1430
Pomiankowski A, Møller AP (1995) A resolution of the lek paradox. Proc R Soc Lond B 260:21–29
Proulx SR (2001) Female choice via indicator traits easily evolves in the face of recombination and migration. Evolution 55:2401–2411
Qvarnstrom A, Brommer JE, Gustafsson L (2006) Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441:84–86
Radwan J (2003) Inbreeding depression in fecundity and inbred line extinction in the bulb mite, Rhizoglyphus robini. Heredity 90:371–376
Radwan J (2004) Effectiveness of sexual selection in removing mutations induced with ionizing radiation. Ecol Lett 7:1149–1154
Radwan J, Unrug J, Śnigórska K, Gawrońska K (2004) Effectiveness of sexual selection in preventing fitness deterioration in bulb mite populations under relaxed natural selection. J Evol Biol 17:94–99
Reid JM, Arcese P, Cassidy ALEV, Hiebert SM, Smith JNM, Stoddard PK, Marr AB, Keller LF (2005a) Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia). Am Nat 165:299–310
Reid JM, Arcese P, Cassidy ALEV, Marr AB, Smith JNM, Keller LF (2005b) Hamilton and Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia). Proc R Soc Lond B 272:481–487
Reinhold K (1998) Sex linkage among genes controlling sexually selected traits. Behav Ecol Sociobiol 44:1–7
Reinhold K (2002) Modelling the evolution of female choice strategies under inbreeding conditions. Genetica 116:189–195
Reinhold K (2004) Modeling a version of the good-genes hypothesis: female choice of locally adapted males. Org Diver Evol 4:157–163
Reinhold K, Engqvist L, Misof B, Kurtz J (1999) Meiotic drive and evolution of female choice. Proc R Soc Lond B 266:1341–1355
Rice WR (1984) Sex chromosomes and the evolution of sexual dimorphism. Evolution 38:735–742
Roff DA (1997) Evolutionary quantitative genetics. Chapman and Hall, New York
Rose MR (1982) Anatagonistic pleiotropy, dominance, and genetic variation. Heredity 48:63–78
Rosenthal GG, Servedio MR (1999) Chase-away sexual selection: resistance to “resistance”. Evolution 53:296–299
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B 263:1415–1421
Rundle HD, Chenoweth SF, Blows MW (2006) The roles of natural and sexual selection during adaptation to a novel environment. Evolution 60:2218–2225
Scribner KT, Smith MH, Johns PE (1989) Environmental and genetic components of antler growth in white-tailed deer. J Mammal 70:284–291
Sheldon BC, Arponen H, Laurila A, Crochet PA, Merila.J. (2003) Sire coloration influences offspring survival under predation risk in the moorfrog. J Evol Biol 16:1288–1295
Sheridan L, Pomiankowski A (1997) Fluctuating asymmetry, spot asymmetry and inbreeding depression in the sexual coloration of male guppy fish. Heredity 79:515–523
Siller S (2001) Sexual selection and the maintenance of sex. Nature 411:689–692
Sinervo B, Lively CM (1996) The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380:240–243
Thomas MA, Klaper R (2004) Genomics for the ecological toolbox. Trends Ecol Evol 19:439–445
Tomkins JL, Radwan J, Kotiaho JS, Tregenza T (2004) Genic capture and resolving the lek paradox. Trends Ecol Evol 19:323–328
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Turelli M, Barton NH (2004) Polygenic variation maintained by balancing selection: pleiotropy, sex-dependent allelic effects and G * E interactions. Genetics 166:1053–1079
Uller T, Eklof J, Andersson S (2005) Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav Ecol Sociobiol 57:584–590
Unrug J, Tomkins J, Radwan J (2004) Alternative phenotypes and sexual selection: can dichotomous handicaps honestly signal quality? Proc R Soc Lond B 271:1401–1406
von Schantz R, Wittzell H, Goransson G, Grahn M, Persson K (1996) MHC genotype and male ornamentation: genetic evidence of the Hamilton-Zuk model. Proc R Soc Lond B 263:265–271
von Schantz T, Wittzell H, Goransson G, Grahn M (1997) Mate choice, male condition-dependent ornamentation and MHC in the pheasant. Hereditas 127:133–140
Wedekind C (2002) Sexual selection and life-history decisions: implications for supportive breeding and the management of captive populations. Conserv Biol 16:1204–1211
Wegner KM, Kalbe M, Kurtz J, Reusch TBH, Milinski M (2003) Parasite selection for immunogenetic optimality. Science 301:1343
Wegner KM, Reush TBH, Kalbe M (2003) Multiple parasites are driving major histocompatibility complex polymorphism in the wild. J Evol Biol 224:224–232
Welch AM (2003) Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57:883–893
Welch AM, Semlitsch RD, Gerhardt HC (1998) Call duration as an indicator of genetic quality in male gray tree frogs. Science 280:1928–1930
Westerdahl H, Hansson. B., Bensch. S., Hasselquist. D (2004) Between-year variation of MHC allele frequencies in great reed warblers: selection or drift? J Evol Biol 17:485–492
Westerdahl H, Waldenstrom J, Hansson B, Hasselquist D, von Schantz T, Bensch S (2005) Associations between malaria and MHC genes in a migratory songbird. Proc R Soc Lond B 271:1511–1518
Young JR, Hupp JW, Bradbury JW, Braun CE. 1994. Phenotypic divergence of secondary sexual traits among Sage Grouse populations. Anim Behav 47:1353–1362
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Zajitschek SRK, Evans JP, Brooks R (2006) Independent effects of familiarity and mating preferences for ornamental traits on mating decisions in guppies. Behav Ecol 17:911–916
Acknowledgements
I thank Hanna Kokko, Joe Tomkins, Suzie Mills and Michael Puurtinen, guest editor Trevor Pritcher and anonymous referees for their comments on the previous versions of the manuscript, and organizers and participants of “The evolutionary ecology of genetic quality symposium” for inspiration and constructive discussions. This work was supported by a grant from the Ministry of Science and Higher Education 0494/P04/2005/28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radwan, J. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134, 113–127 (2008). https://doi.org/10.1007/s10709-007-9203-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9203-0