Abstract
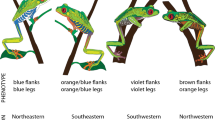
Despite the predicted purifying role of stabilising selection against variation in warning signals, many aposematic species exhibit high variation in their colour patterns. The maintenance of such variation is not well understood, but it has been suggested to be the result of an interaction between sexual and natural selection. This interaction could also facilitate the evolution of sexual dichromatism. Here we analyse in detail the colour patterns of the poison frog Dendrobates tinctorius and evaluate the possible correlates of the variability in aposematic signals in a natural population. Against the theoretical predictions of aposematism, we found that there is enormous intra-populational variation in colour patterns and that these also differ between the sexes: males have a yellower dorsum and bluer limbs than females. We discuss the possible roles of natural and sexual selection in the maintenance of this sexual dimorphism in coloration and argue that parental care could work synergistically with aposematism to select for yellower males.




Similar content being viewed by others
References
Born M, Bongers F, Poelman EH, Sterck FJ (2010) Dry-season retreat and dietary shift of the dart-poison frog Dendrobates tinctorius (Anura: Dendrobatidae). Phyllomedusa 9:37–52
Boukal DS, Berec L, Krivan V (2008) Does sex-selective predation stabilize or destabilize predator-prey dynamics? PLoS One 3:e2687. doi:10.1371/journal.pone.0002687
Brakefield PM (1985) Polymorphic Mullerian mimicry and interactions with thermal melanism in ladybirds and a soldier beetle. A hypothesis. Biol J Linn Soc 26:243–267
Brodie ED (1992) Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution 46:1284–1298
Brodie ED (1993) Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution 47:227–235
Chouteau M, Angers B (2011) The role of predators in maintaining the geographic organization of aposematic signals. Am Nat 178:810–817
Christe P, Keller L, Roulin A (2006) The predation cost of being a male: implications for sex-specific rates of ageing. Oikos 114:381–394
Codella SG, Lederhouse RC (1989) Intersexual comparison of mimetic protection in the black swallowtail butterfly, Papilio polyxenes. Experiments with captive blue jay predators. Evolution 43:410–420
Comeault AA, Noonan BP (2011) Spatial variation in the fitness of divergent aposematic phenotypes of the poison frog, Dendrobates tinctorius. J Evol Biol 24:1374–1379
Crothers L, Gering E, Cummings M (2011) Aposematic signal variation predicts male–male interactions in a polymorphic poison frog. Evolution 65:599–605
Darst CR, Cummings ME, Cannatella DC (2006) A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proc Natl Acad Sci 103:5852–5857
Devillechabrolle J (2011) Mise en place et analyse d’un protocole pour le suivi à long terme d’amphibiens en forêt tropicale humide de Guyane Française. Masters Thesis, University of Marseille, Marseille, France
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton
Endler JA (1988) Frequency-dependent predation, crypsis and aposematic coloration. Philos Trans R Soc Lond B 319:505–523
Endler JA (1991) Interactions between predators and prey. In: Krebs JR, Davies NB (eds) Behavioural ecology. An evolutionary approach. Cambridge University Press, Cambridge, pp 169–196
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Endler JA (2000) Evolutionary implications of the interaction between animal signals and the environment. In: Espmark YO, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic Press, Trondheim, pp 11–46
Endler JA (2012) a framework for analysing colour pattern geometry: adjacent colours. Biol J Linn Soc 107:233–253
Endler JA, Mappes J (2004) Predator mixes and the conspicuousness of aposematic signals. Am Nat 163:532–547
Exnerová A, Svádová K, Stys P, Barcalová S, Landová E, Prokopovvá M, Fuchs R, Socha R (2006) Importance of colour in the reaction of passerine predators to aposematic prey: experiments with mutants of Pyrrhocoris apterus (Heteroptera). Biol J Linn Soc 88:143–153
Forsman A, Appelqvist S (1999) Experimental manipulation reveals differential effects of colour pattern on survival in male and female pygmy grasshoppers. J Evol Biol 12:391–401
Gray SM, McKinnon JS (2007) Linking color polymorphism maintenance and speciation. Trends Ecol Evol 22:71–79
Greenwood JJD, Wood EM, Batchelor S (1981) Apostatic selection of distasteful prey. Heredity 47:27–34
Hegna RH, Saporito RA, Gerow KG, Donnelly MA (2011) Contrasting colors of an aposematic poison frog do not affect predation. Ann Zool Fenn 48:29–38
Joron M, Mallet J (1998) Diversity in mimicry: paradox or paradigm? Trends Ecol Evol 13:461–463
Kunte K (2008) Mimetic butterflies support Wallace’s model of sexual dimorphism. Proc R Soc B 275:1617–1624
Lederhouse RC, Scriber JM (1987) Increased relative frequency of dark morph females in the tiger swallowtail Papilio glaucus (Lepidoptera, Papilionidae) in South-Central Florida. Am Midl Nat 118:211–213
Lescure J, Marty C (2000) Atlas des Amphibiens de Guyane, vol 45. Muséum National D’Histoire Naturelle, Paris
Lindell LE, Forsman A (1996) Sexual dichromatism in snakes: support for the flicker-fusion hypothesis. Can J Zool 74:2254–2256
Lötters S, Jungfer K-H, Henkel FW, Schmidt W (2007) Poison frogs: biology, species and captive husbandry. Edition Chimaira, Frankurt
Maan ME, Cummings ME (2008) Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 62:2334–2345
Maan ME, Cummings ME (2009) Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. Proc Natl Acad Sci 106:19072–19077
Mallet J, Joron M (1999) Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation. Ann Rev Ecol Syst 30:201–233
Myers CW, Daly JW (1983) Dart-poison frogs. Sci Am 248:96–105
Nokelainen O, Hegna RH, Reudler JH, Lindstedt C, Mappes J (2012) Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc R Soc B 279:257–265
Noonan BP, Comeault AA (2009) The role of predator selection on polymorphic aposematic poison frogs. Biol Lett 5:51–54
Noonan BP, Gaucher P (2006) Refugial isolation and secondary contact in the dyeing poison frog Dendrobates tinctorius. Mol Ecol 15:4425–4435
O’Donald P, Majerus MEN (1984) Polymorphism of melanic ladybirds maintained by frequency-dependent sexual selection. Biol J Linn Soc 23:101–111
Poulton EB (1890) The colours of animals: their meaning and use, vol 26. Kegan Paul, Trench
Pröhl H, Ostrowski T (2011) Behavioural elements reflect phenotypic colour divergence in a poison frog. Evol Ecol 25:993–1015
Prudic KL, Oliver JC, Sperling FAH (2007) The signal environment is more important than diet or chemical specialization in the evolution of warning coloration. Proc Natl Acad Sci 104:19381–19386
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rudh A, Rogell B, Hastad O, Qvarnstrom A (2011) Rapid population divergence linked with co-variation between coloration and sexual display in strawberry poison frogs. Evolution 65:1271–1282
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, Oxford
Saporito RA, Zuercher R, Roberts M, Gerow KG, Donnelly MA (2007) Experimental evidence for aposematism in the dendrobatid poison frog Oophaga pumilio. Copeia 2007:1006–1011
Shine R, Madsen T (1994) Sexual dichromatism in snakes of the genus Vipera. A reviews and a new evolutionary hypothesis. J Herpetol 28:114–117
Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K (2004) Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol 207:2471–2485
Silverstone PA (1975) A revision of the poison-arrow frogs of the genus Dendrobates Wagler. Nat Hist Mus Los Angeles Co. Sci Bull 21:1–55
Sinervo B, Svensson E (2002) Correlational selection and the evolution of genomic architecture. Heredity 89:329–338
Stokes AN, Cook DG, Hanifin CT, Brodie ED (2011) Sex-biased predation on newts of the genus Taricha by a novel predator and its relationship with tetrodotoxin toxicity. Am Midl Nat 165:389–399
Summers K, Clough ME (2001) The evolution of coloration and toxicity in the poison frog family (Dendrobatidae). Proc Natl Acad Sci 98:6227–6232
Ueno H, Sato Y, Tsuchida K (1998) Colour-associated mating success in a polymorphic Ladybird Beetle, Harmonia axyridis. Funct Ecol 12:757–761
Valkonen J, Niskanen M, Bjorklund M, Mappes J (2011) Disruption or aposematism? Significance of dorsal zigzag pattern of European vipers. Evol Ecol 25:1047–1063
Williams P (2007) The distribution of bumblebee colour patterns worldwide: possible significance for thermoregulation, crypsis, and warning mimicry. Biol J Linn Soc 92:97–118
Wollenberg KC, Lotters S, Mora-Ferrer C, Veith M (2008) Disentangling composite colour patterns in a poison frog species. Biol J Linn Soc 93:433–444
Acknowledgments
This study was funded by two Les Nouragues grants from the CNRS (France), and student research allowances from the School of Psychology at the University of Exeter (UK) and the CIE at Deakin University (Australia), all to BR. P. Gaucher and M. Fernandez provided logistic support. We are thankful to Diana Pizano and J. Devillechabrolle for assistance in the field, and to J. Mappes, J. Valkonen, J. Brown and two anonymous reviewers for thoughtful comments and suggestions that improved the manuscript. This work was done in compliance with the local environmental regulations (research permit issued by CNRS-Guyane) and following ASAB’s guidelines for the treatment of animals in research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojas, B., Endler, J.A. Sexual dimorphism and intra-populational colour pattern variation in the aposematic frog Dendrobates tinctorius . Evol Ecol 27, 739–753 (2013). https://doi.org/10.1007/s10682-013-9640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-013-9640-4