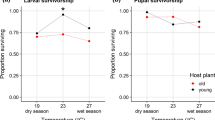
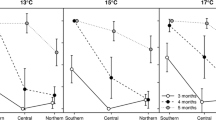
Abstract
Seasonal generations of short-lived organisms often differ in their morphological, behavioural and life history traits, including body size. These differences may be either due to immediate effects of seasonally variable environment on organisms (responsive plasticity) or rely on presumably adaptive responses of organisms to cues signalizing forthcoming seasonal changes (anticipatory plasticity). When directly developing individuals of insects are larger than their overwintering conspecifics, the between-generation differences are typically ascribed to responsive plasticity in larval growth. We tested this hypothesis using the papilionid butterly Iphiclides podalirius as a model species. In laboratory experiments, we demonstrated that seasonal differences in food quality could not explain the observed size difference. Similarly, the size differences are not likely to be explained by the immediate effects of ambient temperature and photoperiod on larval growth. The qualitative pattern of natural size differences between the directly developing and diapausing butterflies could be reproduced in the laboratory as a response to photoperiod, indicating anticipatory character of the response. Directly developing and diapausing individuals followed an identical growth trajectory until the end of the last larval instar, with size differences appearing just a few days before pupation. Taken together, various lines of evidence suggest that between-generation size differences in I. podalirius are not caused by immediate effects of environmental factors on larval growth. Instead, these differences rather represent anticipatory plasticity and are thus likely to have an adaptive explanation. It remains currently unclear, whether the seasonal differences in adult size per se are adaptive, or if they constitute co-product of processes related to the diapause. Our study shows that it may be feasible to distinguish between different types of plasticity on the basis of empirical data even if fitness cannot be directly measured, and contributes to the emerging view about the predominantly adaptive nature of seasonal polyphenisms in insects.




Similar content being viewed by others
References
AEMET (Agencia Estatal de Meteorología) (2011a) Valores climatológicos extremos. Barcelona/aeropuerto, 1971–2000. http://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/efemerides_extremos?w=0&k=cat&l=0076&datos=det
AEMET (Agencia Estatal de Meteorología) (2011b) Valores climatológicos normales. Barcelona/aeropuerto, 1971–2000. http://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/valoresclimatologicos?l=0076&k=cat
Angilletta MJ, Dunham AE (2003) The temperature-size rule in ectotherms: simple evolutionary explanations may not be general. Am Nat 162:332–342
Arendt JD (2011) Size-fecundity relationships, growth trajectories, and the temperature-size rule for ectotherms. Evolution 65:43–51
Atkinson D (1994) Temperature and organism size: a biological law for ectotherms? Adv Ecol Res 25:1–58
Blanckenhorn WU (2009) Causes and consequences of phenotypic plasticity in body size: the case of the yellow dung fly Scathophaga stercoraria (Diptera: Scathophagidae). In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity in insects. Mechanisms and consequences. Science Publishers, Einfield, pp 369–422
Blau WS (1981) Life history variation in the black swallowtail butterfly. Oecologia 48:116–222
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Borges I, Soares AO, Magro A, Hemptinne JL (2011) Prey availability in time and space is a driving force in life history evolution of predatory insects. Evol Ecol 25:1307–1319
Brakefield PM (1996) Seasonal polyphenism in butterflies and natural selection. Trends Ecol Evol 11:275–277
Brakefield PM, Frankino WA (2009) Polyphenisms in Lepidoptera: multidisciplinary approaches to studies of evolution and development. In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity in insects. Mechanisms and consequences. Science Publishers, Einfield, pp 337–368
Brakefield PM, Larsen TB (1984) The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol J Linn Soc 22:1–12
Canfield M, Greene E (2009) Phenotypic plasticity and the semantics of polyphenism: a historical review and current perspectives. In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity in insects. Mechanisms and consequences. Science Publishers, Einfield, pp 65–80
Dominick OS, Truman JW (1984) The physiology of wandering behaviour in Manduca sexta. I. Temporal organization and the influence of the internal and external environments. J Exp Biol 110:35–51
Esperk T, Tammaru T (2004) Does the ‘investment principle’ model explain moulting strategies in lepidopteran larvae? Physiol Entomol 29:56–66
Esperk T, Tammaru T (2010) Size compensation in moth larvae: attention to larval instars. Physiol Entomol 35:222–230
Fischer K, Fiedler K (2001) Sexual differences in life-history traits in the butterfly Lycaena tityrus: a comparison between direct and diapause development. Entomol Exp Appl 100:325–330
Friberg M, Karlsson B (2010) Life-history polyphenism in the Map butterfly (Araschnia levana): developmental constraints versus season-specific adaptations. Evol Ecol Res 12:603–615
Friberg M, Aalberg Haugen IM, Dahlerus J, Gotthard K, Wiklund C (2011) Asymmetric life-history decision-making in butterfly larvae. Oecologia 165:301–310
Fric Z, Konvička M (2002) Generations of the polyphenic butterfly Araschnia levana differ in body design. Evol Ecol Res 4:1017–1032
Gebhardt MD, Stearns SD (1988) Reaction norms for development time and weight at eclosion in Drosophila mercatorum. J Evol Biol 1:335–354
Gebhardt MD, Stearns SD (1993) Phenotypic plasticity for life history traits in Drosophila melanogaster. I. Effect on phenotypic and environmental correlations. J Evol Biol 6:1–16
Gotthard K, Berger D (2010) The diapause decision as a cascade switch for adaptive developmental plasticity in body mass in a butterfly. J Evol Biol 23:1129–1137
Gotthard K, Nylin S (1995) Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos 74:3–17
Greene E (1989) A diet-induced developmental polymorphism in a caterpillar. Science 243:643–646
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hahn DA, Denlinger DL (2007) Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol 53:760–773
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121
Hazel WN (2002) The environmental and genetic control of seasonal polyphenism in larval color and its adaptive significance in a swallowtail butterfly. Evolution 56:342–348
Hazel WN, Ante S, Stringfellow B (1998) The evolution of environmentally-cued pupal colour in swallowtail butterflies: natural selection for pupation site and pupal colour. Ecol Entomol 23:41–44
Jones RE (1992) Phenotypic variation in Australian Eurema species. Aust J Zool 40:371–383
Karlsson B, Johansson A (2008) Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proc R Soc B 275:2131–2136
Karlsson B, Wickman PO (1989) The cost of prolonged life: an experiment on a nymphalid butterfly. Funct Ecol 3:399–405
Kimura T, Masaki S (1977) Brachypterism and seasonal adaptation in Orgyia thyellina Butler (Lepidotera, Lymantriidae). Kontyû 45:97–106
Kingsolver JG, Huey RB (1998) Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am Zool 38:545–560
Kingsolver JG, Huey RB (2008) Size, temperature, and fitness: three rules. Evol Ecol Res 10:251–268
Koštál V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52:113–127
Larsdotter Mellström H, Friberg M, Borg-Karlson A-K, Murtazina R, Palm M, Wiklund C (2010) Seasonal polyphenism in life history traits: time costs of direct development in a butterfly. Behav Ecol Sociobiol 64:1377–1383
Leather SR, Walters KFA, Bale JS (1993) The ecology of insect overwintering. Cambridge University Press, Cambridge
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute, Cary
Liu ZD, Gong PY, Wu KJ, Wei W, Sun JH, Li DM (2007) Effects of larval host plants on over-wintering preparedness and survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J Insect Physiol 53:1016–1026
Nealis VG (2012). The phenological window for western spruce budworm: seasonal decline in resource quality. Agric Forest Entomol (in press)
Nelson RJ, Denlinger DL, Somers DE (eds) (2010) Photoperiodism: the biological calendar. Oxford University Press, Oxford
Nijhout HF (1999) Control mechanisms of polyphenic development in insects—in polyphenic development, environmental factors alter some aspects of development in an orderly and predictable way. Bioscience 49:181–192
Nijhout HF (2003) Development and evolution of adaptive polyphenisms. Evol Dev 5:9–18
Ohgushi T (1996) Consequences of adult size for survival and reproductive performance in a herbivorous ladybird beetle. Ecol Entomol 21:47–55
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. John Hopkins University Press, Baltimore
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2012) nlme: linear and nonlinear mixed effects models. R package version 3.1-103. http://www.R-project.org
Rehan SM, Schwarz MP, Richards MH (2011) Fitness consequences of ecological constraints and implications for the evolution of sociality in an incipiently social bee. Biol J Linn Soc 103:57–67
Remmel T, Tammaru T, Mägi M (2009) Seasonal mortality trends in tree-feeding insects: a field experiment. Ecol Entomol 34:98–106
Rodrigues D, Moreira GRP (2004) Seasonal variation in larval host plants and consequences for Heliconius erato (Lepidoptera: Nymphalidae) adult body size. Aust Ecol 29:437–445
Schroeder LA (1986) Changes in tree leaf quality and growth-performance of lepidopteran larvae. Ecology 67:1628–1636
Scriber JM (1994) Climatic legacies and sex chromosomes: latitudinal patterns of voltinism, diapause, body size, and host-plant selection on two species of swallowtail butterflies at their hybrid zone. In: Danks HV (ed) Insect life-cycle polymorphism: theory, evolution and ecological consequences for seasonality and diapause control. Kluwer, Dordrecht, pp 133–171
Scriber JM, Slansky F Jr (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211
Shapiro AM (1976) Seasonal polyphenism. Evol Biol 9:259–333
Stefanescu C (2004) Seasonal change in pupation behaviour and pupal mortality in a swallowtail butterfly. Anim Biodiv Cons 27:25–36
Stefanescu C, Pintureau B, Tschorsnig HP, Pujade-Villar J (2003) The parasitoid complex of the butterfly Iphiclides podalirius feisthamelii (Lepidoptera: Papilionidae) in North-East Spain. J Nat Hist 37:379–396
Stefanescu C, Jubany J, Dantart J (2006) Egg-laying by the butterfly Iphiclides podalirius (Lepidoptera, Papilionidae) on alien plants: a broadening of host range or oviposition mistakes? Anim Biodiv Cons 29:83–90
Tammaru T (1998) Determination of adult size in a folivorous moth: constraints at instar level? Ecol Entomol 23:80–89
Tammaru T, Esperk T (2007) Growth allometry of immature insects: larvae do not grow exponentially. Funct Ecol 21:1099–1105
Tammaru T, Ruohomäki K, Saloniemi I (1999) Within-season variability of pupal period in the autumnal moth: a bet-hedging strategy? Ecology 80:1666–1677
Tanaka K, Tsubaki Y (1984) Seasonal dimorphism, growth and food consumption in the swallowtail butterfly Papilio xuthus. Kontyû 52:390–398
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford
Teder T, Tammaru T (2005) Sexual size dimorphism within species increases with body size in insects. Oikos 108:321–334
Teder T, Esperk T, Remmel T, Sang A, Tammaru T (2010) Counterintuitive size patterns in bivoltine moths: late-season larvae grow larger despite lower food quality. Oecologia 162:117–125
Tolman T, Lewington R (2008) Collins butterfly guide. The most complete guide to the butterflies of Britain and Europe. HarperCollins, London
Van Asch M, Visser ME (2007) Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol 52:37–55
Van Dyck H, Wiklund C (2002) Seasonal butterfly design: morphological plasticity among three developmental pathways relative to sex, flight and thermoregulation. J Evol Biol 15:216–225
Wang X, Yang Q, Zhou X, Zhao F, Lei C (2007) Effect of photoperiod associated with diapause induction on the accumulation of metabolites in Sericinus montelus (Lepidoptera: Papilionidae). Appl Entomol Zool 42:419–424
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important? In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity in insects. Mechanisms and consequences. Science Publishers, Einfield, pp 1–63
Wiklund C (1971) Inonhusodling av makaonfjärilen. Zool Revy 33:35–42
Wiklund C, Nylin S, Forsberg J (1991) Sex-related variation in growth-rate as a result of selection for large size and protandry in a bivoltine butterfly, Pieris napi. Oikos 60:241–250
Windig JJ, Brakefield PM, Reitsma N, Wilson JGM (1994) Seasonal polyphenism in the wild: survey of wing patterns in five species of Bicyclus butterflies in Malawi. Ecol Entomol 19:285–298
Acknowledgments
We are grateful to Anu Tiitsaar, Freerk Molleman, Robert B. Davis, Juhan Javoiš and three anonymous referees for constructive comments on the manuscript. Kristiina Ehapalu, Jordi Jubany, Taavet Kukk, Aigi Margus, Kristin Markov, Marta Miralles, and Martin Sauk provided technical help. The municipal council of Sant Celoni provided all facilities to carry out the food quality experiment. The study was supported by the Estonian Science Foundation grants 7406, 8413 and 9294, the targeted financing project SF0180122s08, and by the European Union through the European Regional Development Fund (Center of Excellence FIBIR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esperk, T., Stefanescu, C., Teder, T. et al. Distinguishing between anticipatory and responsive plasticity in a seasonally polyphenic butterfly. Evol Ecol 27, 315–332 (2013). https://doi.org/10.1007/s10682-012-9598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9598-7