Abstract
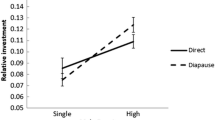
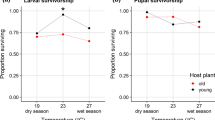
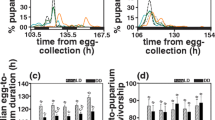
Insects with two or more generations per year will generally experience different selection regimes depending on the season, and accordingly show seasonal polyphenisms. In butterflies, seasonal polyphenism has been shown with respect to morphology, life history characteristics and behaviour. In temperate bivoltine species, the directly developing generation is more time-constrained than the diapause generation, and this may affect various life history traits such as mating propensity (time from eclosion to mating). Here, we test whether mating propensity differs between generations in Pieris napi, along with several physiological parameters, i.e. male sex pheromone synthesis, and female ovigeny index and fecundity. As predicted, individuals of the directly developing generation—who have shorter time for pupal development—are more immature at eclosion; males take longer to synthesise the male sex pheromone after eclosion and take longer to mate than diapause generation males. Females show the same physiological pattern; the directly developing females lay fewer eggs than diapausing females during the first days of their life. Nevertheless, the directly developing females mate faster after eclosion than diapausing females, indicating substantial adult time stress in this generation and possibly an adaptive value of shortening the pre-reproductive period. Our study highlights how time stress can be predictably different between generations, affecting both life history and behaviour. By analysing several life history traits simultaneously, we adopt a multi-trait approach to examining how adaptations and developmental constraints likely interplay to shape these seasonal polyphenisms.




Similar content being viewed by others
References
Abrams PA, Leimar O, Nylin S, Wiklund C (1996) The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am Nat 147:381–395
Andersson J, Borg-Karlson AK, Vongvanich N, Wiklund C (2007) Male sex pheromone release and female mate choice in a butterfly. J Exp Biol 210:964–970
Bergström J, Wiklund C (2005) No effect of male courtship intensity on female remating in the butterfly Pieris napi. J Insect Behav 18:479–489
Bergström J, Wiklund C, Kaitala A (2002) Natural variation in female mating frequency in a polyandrous butterfly: effects of size and age. Anim Behav 64:49–54
Breuker CJ, Brakefield PM (2002) Female choice depends on size but not symmetry of dorsal eyespots in the butterfly Bicyclus anynana. Proc R Soc Lond B 269:1233–1239
Costanzo K, Monteiro A (2007) The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proc R Soc Lond B 274:845–851
Fischer K, Perlick J, Galetz T (2008) Residual reproductive value and male mating success: older males do better. Proc R Soc Lond B 275:1517–1524
Forsberg J, Wiklund C (1988) Protandry in the green-veined white butterfly, Pieris napi. L. (Lepidoptera; Pieridae). Funct Ecol 2:81–88
Friberg M, Wiklund C (2007) Generation-dependent female choice: behavioral polyphenism in a bivoltine butterfly. Behav Ecol 18:758–763
Gotthard K, Nylin S, Wiklund C (1999) Seasonal plasticity in two satyrine butterflies: state-dependent decision making in relation to daylength. Oikos 84:453–462
Iwasa Y, Odendaal FJ, Murphy DD, Ehrlich PR, Launer AE (1983) Emergence patterns in male butterflies—a hypothesis and a test. Theor Popul Biol 23:363–379
Jervis MA, Ferns PN (2004) The timing of egg maturation in insects: ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 107:449–460
Johnstone RA (1997) The tactics of mutual mate choice and competitive search. Behav Ecol Sociobiol 40:51–59
Kaitala A, Wiklund C (1995) Female mate choice and mating costs in the polyandrous butterfly Pieris napi (Lepidoptera, Pieridae). J Insect Behav 8:355–363
Karlsson B, Johansson A (2008) Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proc R Soc Lond B 275:2131–2136
Karlsson B, Stjernholm F, Wiklund C (2008) Test of a developmental trade-off in a polyphenic butterfly: direct development favours reproductive output. Funct Ecol 22:121–126
Kemp DJ (2007) Female butterflies prefer males bearing bright iridescent ornamentation. Proc R Soc Lond B 274:1043–1047
Kemp DJ (2008) Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae). Behav Ecol 19:1–8
Kokko H, Mappes J (2005) Sexual selection when fertilization is not guaranteed. Evolution 59:1876–1885
Larsdotter Mellström H, Wiklund C (2009) Males use sex pheromone assessment to tailor ejaculates to risk of sperm competition in a butterfly. Behav Ecol 20:1147–1151
Nieberding CM, de Vos H, Schneider MV, Lassance J-M, Estramil N, Andersson J, Bång J, Hedenström E, Löfstedt C, Brakefield PM (2008) The male sex pheromone of the butterfly Bicyclus anynana: towards an evolutionary analysis. PLoS ONE 3(7):e2751
Nylin S, Wickman PO, Wiklund C (1989) Seasonal plasticity in growth and development of the Speckled Wood butterfly, Pararge aegeria (Satyrinae). Biol J Linn Soc 38:155–171
Papke RS, Kemp DJ, Rutowski RL (2007) Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L. (Pieridae) (vol 73, pg 47, 2007). Anim Behav 73:1083–1083
Robertson KA, Monteiro A (2005) Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc R Soc Lond B 272:1541–1546
R Development Core Team (2008) R Version 2.6.2, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
StatSoft, Inc. (2008) STATISTICA for Windows (data analysis software system), version 8.0. www.statsoft.com
Vanewright RI, Boppre M (1993) Visual and chemical signalling in butterflies—functional and phylogenetic perspectives. Proc R Soc Lond B 340:197–205
Välimäki P, Kaitala A (2007) Life history tradeoffs in relation to the degree of polyandry and developmental pathway in Pieris napi (Lepidoptera, Pieridae). Oikos 116:1569–1580
Välimäki P, Kaitala A, Kokko H (2006) Temporal patterns in reproduction may explain variation in mating frequencies in the green-veined white butterfly Pieris napi. Behav Ecol Sociobiol 61:99–107
Välimäki P, Kivela SM, Jaaskelainen L, Kaitala A, Kaitala V, Oksanen J (2008) Divergent timing of egg-laying may maintain life history polymorphism in potentially multivoltine insects in seasonal environments. J Evol Biol 21:1711–1723
Wiklund C (2003) Sexual selection and the evolution of butterfly mating systems. In: Boggs CL, Watt W, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. Chicago University Press, Chicago, pp 67–90
Wiklund C, Fagerström T (1977) Why do males emerge before females—hypothesis to explain incidence of protandry in butterflies. Oecologia 31:153–158
Wiklund C, Solbreck C (1982) Adaptive versus incidental explanations for the occurrence of protandry in a butterfly, Leptidea sinapis L. Evolution 36:56–62
Acknowledgements
We thank Birgitta Tullberg, Bengt Karlsson and two anonymous reviewers for constructive comments on the manuscript. This work was supported by the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Wedell
Rights and permissions
About this article
Cite this article
Larsdotter Mellström, H., Friberg, M., Borg-Karlson, AK. et al. Seasonal polyphenism in life history traits: time costs of direct development in a butterfly. Behav Ecol Sociobiol 64, 1377–1383 (2010). https://doi.org/10.1007/s00265-010-0952-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0952-x