Abstract
Zooplankton is widely recognized as a key component of pelagic ecosystems and forms the basis for major trophic webs. Although zooplankton has often been used as an indicator of trophic state, it has not been included as an obligatory element of the water assessment systems compliant with the Water Framework Directive. This article introduces the Zooplankton Index for Polish Lakes’ Assessment (ZIPLAS) as a new method to assess the ecological status of stratified lakes based on the zooplankton community. The ZIPLAS evaluates three aspects of zooplankton communities, namely, taxonomic composition and abundance, diversity of the zooplankton community, and stressor-sensitive species, which are combined into a multimetric index. Following are the metrics used to compose multimetric ZIPLAS: percentage share of the Rotifer species indicative of high trophy in the indicative group’s number (IHTROT; %), ratio of Calanoida to Cyclopoida individual numbers (CA/CY), percentage of tecta form in the population of Keratella cochlearis (TECTA; %), Margalef’s index (d), and zooplankton abundance (NZOL; ind./L). ZIPLAS responds clearly to eutrophication indicators—the strongest with Secchi disc visibility (Spearman’s rank correlation R = 0.86) and slightly weaker with the expressed by total phosphorus (R = -0.74), total nitrogen (R = 0.68) and the catchment pressure expressed by the nutrient loads generated by different types of land use (R = -0.58).
Similar content being viewed by others
Introduction
Zooplankton community is composed of small organisms (Crustacea and Rotifera) passively floating within the water column (or having only slight movement ability) inhabiting oceans, seas, and freshwaters, including lakes. Plankton animals play an important role in the functioning of aquatic ecosystems due to their position in the trophic chain. They are a valuable food source for planktivorous fish (top-down control) and feed on phytoplankton, controlling algae population (bottom-up control; Jeppesen et al., 2011).
Eutrophication is one of the major threats to European surface waters, including Poland. The initial stage of the eutrophication process stimulates the biological production and results in an increase in the number of fish. After exceeding a certain threshold of nutrient concentration, secondary effects of this process are observed. One of the most apparent effects of eutrophication is the massive development of planktic algae that creates mass blooms in the surface water layer, which reduces the water transparency (Lampert & Sommer, 2001) The limitation of light by phytoplankton causes the displacement of macrophytes and indirectly leads to the reconstruction of the composition of the accompanying fauna. In advanced eutrophication phase, oxygen depletion is usually observed in the bottom layer, which leads to the disappearance of fauna (e.g., sensitive relict species), including mass mortality of fish.
Eutrophication also affects the composition and abundance of zooplankton community. In the course of water nutrient enrichment, large Cladocerans are replaced by smaller ones (Jeppesen et al., 2000); this is the most evident effect of eutrophication. In pelagic zones of eutrophic lakes, small-bodied Cladoceran species such as Bosmina spp. and Chydorus sphaericus are generally more abundant than large-bodied species such as Daphnia spp. (DeMott & Kerfoot, 1982). Generally, low biomass of zooplankton is observed in oligotrophic lakes, which contain a great variety of species, while in lakes of advanced trophy, a large biomass with fewer species has been noted (Gannon & Stemberger, 1978). Under conditions of nutrient enrichment, the average size of zooplankton species often decreases, as smaller species with simpler life cycles and higher rates of reproduction become more abundant in the plankton community (Gliwicz, 1969). Zooplankton taxa have different preferences for trophic state (Berzins & Bertilsson, 1989; Berzins & Pejler, 1989; Lougheed & Chow-Fraser, 2002) and water clarity. Obviously, many species occur in lakes with various trophies, but within Crustacean and Rotifer communities, some species prefer high or low trophic waters.
Deterioration of the conditions in a lake adversely impacts features of zooplankton community; for example, it leads to increased biomass and abundance (Hanson & Peters, 1984), decrease in body size (Karpowicz et al., 2020; Pace, 1986), and reduction in species diversity (Andronikova, 1996; Haberman & Haldna, 2014). Therefore, zooplankton could be a potentially effective indicator to assess small changes in water quality, especially those caused by eutrophication. Comprehensive studies demonstrate the use of zooplankton as an effective eutrophication indicator (Andronikova, 1996; Carpenter et al., 2006; Čeirāns, 2007; De-Carli et al., 2019; Dembowska et al., 2015; Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013; Haberman & Haldna, 2014; Karabin, 1985; Karpowicz et al., 2020; Ochocka & Pasztaleniec, 2016). Nevertheless, these research studies address trophic categories provided by the Organisation for Economic Cooperation and Development (OECD, 1982), not ecological status classes sensu the Water Framework Directive (WFD, European Commission (EC), 2000).
The WFD introduced a new approach to water quality assessment, which does not refer directly to traditional trophic categories. It refers to the concept of ecological status, understood as ecosystem health, and departs from the traditional approach that employs static water trophic categories. In this concept, naturally eutrophic conditions (without the influence of anthropogenic pressure) are considered as an acceptable state, while eutrophication refers to undesirable effects of nutrient load, resulting from anthropogenic pressure (Soszka, 2009). Consequently, mesotrophic lake, when anthropogenically impacted and altered in relation to natural status, may represent deteriorated ecological status (worse than good), whereas eutrophic lake slightly deviated from natural conditions may represent good ecological status. Thus, while assessing ecological status, it is pivotal to establish type-specific reference conditions, which constitute a benchmark for evaluation of the deviation of the current state from the state expected under undisturbed conditions.
The WFD operates with five classes of ecological status, assessed by using the following biotic elements: phytoplankton, macrophytes and phytobenthos, invertebrates, and fish, and supporting physicochemical and hydromorphological elements. These elements help assess primarily the impact of eutrophication on aquatic ecosystems, and, to a lesser extent the hydromorphological pressure (Poikane et al., 2020). During the last two decades, in all the EU countries, new type-specific WFD-compliant biological methods of ecological status assessment have been elaborated on. In Poland, lake assessment methods based on phytoplankton (Hutorowicz & Pasztaleniec, 2014), macrophytes (Ciecierska & Kolada, 2014), phytobentos (Zgrundo et al., 2020), macroinvertebrates (Bielczyńska et al., 2020), and ichthyofauna (Adamczyk & Prus, 2020) have been implemented under the purview of state monitoring program. However, zooplankton has not been included as one of the obligatory biological quality elements recommended in the WFD. The only mention of this biological element appears in the WFD Monitoring Guidelines elaborated by Working Group within the Common Implementation Strategy (CIS, 2003) supporting WFD implementation, where its analysis has been limited to the role of a “supporting/interpretative parameter” in the assessment of lakes based on fish assemblages.
The reason for this omission remains unclear (Caroni & Irvine, 2010), particularly as zooplankton, has been traditionally involved in the ecological research of lakes in many places, in Russia (Andronikova, 1996), North America (Kane et al., 2009), and in Europe, e.g., Poland (Karabin, 1985; Radwan & Popiołek, 1989), Czechoslovakia (Sládeček, 1983), Sweden (Pejler, 1983), Finland (Hakkari, 1972), the Netherlands (Gulati, 1983), Denmark (Jeppesen et al., 2000), Estonia (Haberman & Haldna, 2014), and Greece (Stamou et al., 2019). Moreover, zooplankton has been used for decades as a bioindicator for routine lake monitoring in Austria, Denmark, Finland, the Netherlands, and Norway (European Environment Agency (EEA), 1996). Zooplankton seems to be a promising indicator for the assessment of the ecological status of lake ecosystems due to its vulnerability to the effects of anthropogenically induced eutrophication and the relatively easy determination of species in contrast to phytoplankton (Ejsmont-Karabin, 2012; Ochocka & Pasztaleniec, 2016).
The failure to include zooplankton as part of the assessment of the ecological status of lakes has been emphasized by Moss (2007). In the scientific literature, the need for the inclusion of zooplankton in the assessment of lake water quality is gaining increased attention (Caroni & Irvine, 2010; Ejsmont-Karabin, 2012; Jeppesen et al., 2011; Karpowicz et al., 2020; Ochocka & Pasztaleniec, 2016).
This study aimed to develop a zooplankton-based index and assessment system to evaluate the ecological status of deep, stratified lakes, in the context of the WFD approach. The present work comprises (1) the selection of candidate zooplankton metrics and their testing in a pressure gradient; (2) the establishment of reference conditions for temperate lowland, stratified lakes; (3) the development of the multimetric index ZIPLAS; (4) testing of the multimetric’s response along the pressure gradient (eutrophication indicators); and (5) setting of the class boundary values for ZIPLAS. Since no specific guidelines for elaborating zooplankton multimetric exists, general guidelines for defining biological WFD-compliant metrics and the criteria for the selection of multimetric components for aquatic invertebrates were adopted (Hering et al., 2006). The ZIPLAs multimetric consists of five metrics that take into account the composition and abundance and the diversity and occurrence of sensitive taxa. Compared to single-metrics indices, multimetric indices act as a complex tool for assessing water ecosystems, since they integrate different stressors and components of the community (Hering et al., 2006). To meet the criteria for a reliable assessment metric, an index should respond significantly and directionally to pressure.
Material and methods
Study area
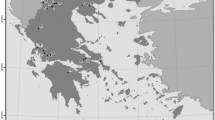
Zooplankton samples were collected from 45 lakes located in north-eastern Poland (see Fig. 1) during the summer period (July–August), of the years 2012–2015 while conducting the dedicated research projects.
Localization of studied lakes in Masurian (dark gray color) and Lithuanian (light gray color) Lakelands. The black, dotted line shows the area of the last Baltic glaciation; the gray line shows the largest rivers in Poland. The numbers refer to the lake names: 1—Blizno; 2—Busznica, 3—Kalejty, 4—Sajno; 5—Olecko Małe; 6—Rajgrodzkie; 7—Łaśmiady; 8—Gawlik; 9—Garbaś Mały; 10—Zdrężno; 11—Niegocin; 12—Buwełno; 13—Boczne; 14—Jagodne; 15—Ryńskie; 16—Majcz Wielki; 17—Kuc; 18—Mikołajskie; 19—Nidzkie; 20—Lampackie; 21—Piłakno; 22—Gant; 23—Jegocin; 24—Roś; 25—Omulew; 26—Świętajno; 27—Maróz; 28—Bartąg; 29—Ukiel; 30—Kortowskie; 31—Dadaj; 32—Tumiańskie; 33—Kierźlińskie; 34—Leleskie; 35—Kalwa; 36—Purda; 37—Linowskie; 38—Wadąg; 39—Czos; 40—Probarskie; 41—Kiersztanowskie; 42—Kruklin; 43—Brożane; 44—Wiłkokuk; 45—Zelwa
Seven lakes were investigated three times, 27 lakes were inestigated two times, and 11 lakes were investigated once during this period, resulting in 86 lake-years including repetitions. In the temperate zone, the summer stagnation is a stable period when changes in the abiotic and biotic environmental conditions are less. During this period, zooplankton communities are most diverse and attain the highest abundance level (Karabin, 1985). All of the analyzed lakes are lowland (< 200 m a.s.l.), with a surface area ranging from 0.391 to 26 km2 and with alkaline water (> 1.0 meq/L). They are deep, stratified water bodies with a mean depth ranging from 4 to 13 m and a maximum depth ranging from 12 to 57 m (see Table SI 1).
Data collection
The sampling points were located close to the deepest part of each lake. The samples for chemical and zooplankton analyses were taken using a 2.6-L Limnos sampler at intervals of 1-m depth from the surface to the bottom of the epilimnion layer. Water was filtered using a plankton net with a 30-µm mesh size and preserved with Lugol’s solution and 4% formalin. Secchi disc visibility (SD) was measured and field measurements of water temperature, pH, conductivity, and oxygen concentration were carried out using a YSI 6600 V2 multiparametric probe (Ohio, USA). The chemical analyses of total phosphorus (TP) and total nitrogen (TN) concentration were performed in a laboratory, using standard methods (Hermanowicz et al., 1999). The measurement of chlorophyll a concentration was performed by a spectrophotometric method (Nusch, 1980).
Cladocerans and Rotifers were identified to species level. Copepods were divided into nauplii and copepodites, which were identified to order level, while adult copepods were identified to species level. The Crustacean zooplankton biomass was estimated based on the relationship between the body length and body weight for each species, as proposed by Balushkina and Vinberg (1979). The standard wet weight of Rotifers was determined from the individual body weights, as suggested by Ejsmont-Karabin (1998). The species Asplanchna priodonta and Leptodora kindtii were excluded from the analysis because of their large size, which was many times greater than that of both Rotifer and Crustacean species.
Elaboration of the new zooplankton method
The essential step in elaborating the WFD-compliant ecological status assessment system is to establish reference conditions. Reference lakes were defined based on spatially approach (“the best of existing”), where data from undisturbed or minimally disturbed lakes with only slight human disturbances are analyzed. This approach is among the ones recommended by the WFD and has been used in other studies of such kind (e.g., Birk et al., 2012; Lyche-Solheim, 2005; Soszka et al., 2008).
To assign a lake as a reference, the following criteria were applied:
-no point sources of pollution in the total catchment.
-natural land use in the catchment (> 80% area of forests or wetlands, lack of villages in direct contact with the shoreline, no urban areas)
-lack or no intensive recreational use.
-high/good water quality according to existing data.
The data on water quality indicators, which were used to select the reference lakes, came from the Polish State Environmental Monitoring (SEM) program; these data were obtained in the years 2009–2012.
The impact of catchment use on the quality of lake waters was analyzed based on the CORINE Land Cover 2018 (CLC18; Büttner & Kosztra, 2017). The area (km2) occupied by various forms of land use was calculated for each lake. Further, theoretical loads of nitrogen and phosphorus generated by individual forms of land use were calculated using unit values of surface runoff for the individual land use category in the total catchment (Arciszewski et al., 2010). The values of unit loads are presented in Table 1.
To quantify the pressure caused by different forms of land use in the catchment area, for each lake, the cumulative nutrient load index (PCATOT) was calculated, based on the principal component analysis performed in the MVSP software (Kovach, 2007). The PCATOT index used the values of correlation coefficients of the first component axis (PC1) from theoretical TP and TN loads, calculated per unit of water volume (P/V, N/V; Kutyła, 2020). Ultimately, TP, TN, and SD as well as the PCATOT were adopted as parameters of pressure proxies. Based on extensive literature reviews (Andronikova, 1996; Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013; Karabin, 1985; Karpowicz et al., 2020; Margalef, 1958; Shannon & Weaver, 1963), a list of 31 candidate zooplankton indices was selected, which can be sub-divided into three groups based on following characteristics: (1) the composition and abundance of fauna, (2) the diversity of the zooplankton community, and (3) occurrence of sensitive taxa (see Table 2). These indices were tested against proxies of eutrophication parameters (TP, TN, SD) for their response to eutrophication pressure, and best responding metrics within each group were selected to compose the multimetric index (see Table SI 2).
Both the pressure parameters and the tested zooplankton indices did not show a normal distribution; therefore, for all the analyses, the non-parametric statistical tests were used. To select indicators that are sensitive to the intensity of eutrophication, the relationship between the values of the indicators and the parameters of water quality was investigated using Spearman’s rank correlation coefficient method. The indicators that responded well to pressure were those that first showed a statistically significant correlation with the concentration of TP, which is commonly considered as the basic indicator of eutrophication (Lyche-Solheim et al., 2013) and with which the parameters of the zooplankton community are often correlated (Jeppesen et al., 2011; Sondergaard et al., 2005). The threshold value of Spearman’s correlation coefficient (to consider a metric as the one responding well to pressure) was set as R > 0.55. Among the 31 tested indices, those that most strongly correlated with pressure (R > 0.55; see Table SI 2) and represented all metric groups were considered as components of the ZIPLAS multimetric index. The Water Framework Directive introduced the concept of a “metric,” so in this work, indices selected to create the ZIPLAs multimetric were referred as „metrics”. The multicollinearity among the selected metrics was assessed by examining tolerance and variance inflation factor (VIF). The values of zooplankton metrics (which have different units) selected for use in the ZIPLAS were normalized to ecological quality ratios (EQRs), ranging from 0 (the worst status) to 1 ( the best status), using the following equation (Hering et al., 2006):
For indices decreasing with increasing pressure:
For indices increasing with increasing pressure:
Values > 1 were set to 1, while values < 0 were set to 0.
To analyze the response of the selected indices to eutrophication expressed by TP concentration, scatter plots based on lowess smoothed models were used.
The ZIPLAS multimetric is the arithmetic mean of the values of its compositional metrics.
The boundary values for five ecological status classes, i.e., high (H), good (G), moderate (M), poor (P), and bad (B), were determined based on the distribution of ZIPLAS values in the studied lakes. The high/good class boundary (H/G) was set as the 25th percentile of ZIPLAS values for reference lakes, as recommended by Hering et al. (2006). The other boundaries were established using the subsequent percentages of the H/G limit value of ZIPLAS: 75% for G/M, 50% for M/P, and 25% for the P/B boundary. The performance of the ZIPLAS along nutrient gradients was tested using Spearman’s rank correlation test. To show the statistical differentiation of ZIPLAS among ecological classes, the non-parametric Mann–Whitney U test was conducted. All statistical analyses were carried out using STATISTICA 12.0 PL software (StatSoft Inc., 2014).
Results
Environmental characteristics
The parameters of water quality in analyzed lakes indicated trophic conditions, ranging from mesotrophy to hypertrophy (see Table SI 1). In 11 of the 45 lakes, the summer TP values exceeded 60 ug/L, indicating high fertility and hypertrophic conditions, which was reflected in the low values of SD, ranging from 0.8 to 1.8 m. In seven of the least fertile lakes, the TP concentration was less than 20 ug/l, and the chlorophyll content ranged from 1.9 to 10.8 ug/L. These lakes had the highest SD, ranging from 3 to 7.2 m.
Catchment impact
The total catchment area of the examined lakes ranged from 3.6 to 30,303.0 km2. In the case of 12 lakes, the total catchment area was forested by more than 50%. For seven of these lakes, this share exceeded 80% of the catchment area. The analyzed pool of lakes included 12 lakes located in agricultural catchments, where arable land occupied more than half of the total catchment area. The use of the catchment area of other lakes was diversified without a clear dominance of one of the analyzed categories of land use. The values of theoretical phosphorus loads generated in the catchments ranged from 63.0 to 51,487.0 kgP/year and nitrogen loads from 1550.0 to 1,226,983.0 kgN/year. The total catchment area and its type of use influenced the size of nutrient loads entering the lakes.
The phosphorus load potentially generated in the total catchment per unit of water volume ranged from 0.003 to 1.090 gP/m3, and the nitrogen load ranged from 0.07 to 26.70 gN/m3. The values of the PCATOT index ranged from -0.33 (low pressure) to 2.60 (high pressure).
Reference conditions
Six of 45 analyzed lakes were indicated as references based on the pressure criteria. Maximum depth of lakes that were designated as reference ranged from 12 to 48 m, while mean depth ranged from 3.8 to 9 m. Natural land use in the total catchments area ranged from 91 to 100%. Considering the trophic parameters, for the majority of lakes, the concentration of TP rarely exceeded 30 ug/l. Lakes Zelwa and Wiłkokuk investigated in 2013 were exceptions, where higher values have been reported: 46 ug/l and 54 ug/l, respectively. TN concentrations ranged from 0.15 to 0.94 mg/l. Transparency expressed by SD ranged from 2 to 7 m (see Table SI 1). In all reference lakes, 65 species of zooplankton were identified, among which, 28 belonged to Crustacea and 36 to Rotifera. In the Crustacean community, Cladocera was the dominant group. The most frequent species among Crustaceans were Diaphanosoma brachyurum (18%), Daphnia cucullata (13%; which is an indicator species of low-trophic lakes), Eubosmina crassicornis (11%), and Eudiaptomus graciloides (11%). Following species are typical for low-trophic polish lakes—Daphnia cristata, Daphnia galeata, Daphnia hyalina, Eubosmina coregoni, Bythothrepes longimanus, and Heterocope appendiculata—were found in waters of these lakes (Ejsmont-Karabin & Karabin, 2013). In the Rotifer community, Keratella cochlearis occurred most frequently (40%), which is a species commonly found in all types of water. Low-trophic species had a large share in the abundance of Rotifer community: Polyarthra major (11%), Conochilus unicornis (5%), Gastropus stylifer (5%), and Ascomorpha ecaudis (3%).
Development of a multimetric index
Among all of the tested indices, five among the ones that were most strongly correlated with TP, TN, SD, and PCATOT were selected (see Table 3).
These metrics have different ranges of values and different directions of action; therefore, before they were combined in the multimetric index, their values were normalized according to the formulas given in Eqs. 1–5:
The response of normalized values of these indices (ranging from 0 to 1) to TP concentration between 10 and 100 µg/L varied considerably (see Fig. 2).
The lowess smoothed model regression lines showed nonlinearity for five metrics in the analyzed spectrum of TP; only in the case of NZOL, the relationship approximated the linear model. Below the concentration of 10 µgP/L most indices were close to 1.0, indicating reference conditions, and only Margalef’s index reached a value of 0.6. In the TP range from 10 to 30–45 µg/L, the values of CA/CY and IHTROT decreased rapidly from 1.0 to 0.2, whereas in higher TP concentrations, no response was observed. The values of NZOL gradually decreased from 1.0 to 0.6 with increasing TP concentration, throughout the trophic gradient. The values of Margalef’s index systematically decreased (within the range from 0.6 to 0.2) in the TP ranging from 10 to 70 µg/L. Beyond TP value of 70 µg/L, the curves of most indices (except for NZOL) flattened out but at different levels, in the range from 0.3 to below 0.1. TECTA values slightly changed at low TP concentrations (below 45 µg/L), whereas at more than 45 µg/L, the values decreased rapidly (from about 0.9 to 0.3) until the threshold value of 70 µg/L was reached.
The normalized values of selected metrics were combined into ZIPLAS multimetric index (arithmetic average; Eq. 6):
The ZIPLAS showed strong correlations with all pressure parameters—tested, the strongest and positive correlations were observed with SD (R = 0.86; p < 0.0001), while slightly weaker and negative correlations with TP, TN, and PCATOT (R = -0.74, R = -0.68 and R = -0.58; p < 0.0001, respectively). The relationship between the ZIPLAS multimetric and selected proxies of eutrophication (TP and SD) are shown in Fig. 3.
Boundary setting of ecological status classes.
The distribution of ZIPLAS values in investigated lakes was analyzed to determine boundary values for the ecological status classes. The H/G class boundary was thus set at 0.755. In the other classes, boundaries were set by dividing the range of ZIPLAS values between the H/G boundary into four (see Table 4).
Based on the developed boundaries of ZIPLAS classes, 20 lake-years were assessed as high, 25 as good, 18 as moderate, 13 as poor, and 10 as bad.
The distribution of ZIPLAS values across ecological status classes differed significantly (see Fig. 4).
Distribution of TP (A), TN (B), and SD (m) (C) in lakes classified to one of the five classes of ecological status according to the ZIPLA index. High (N = 20), Good (N = 25), Moderate (N = 18), Poor (N = 13), Bad (N = 10); Boxplots: 25–75th percentiles with median, whiskers: range, circles: outliers, stars: extreme values. The level of confidence in comparison of distribution of nutrients between subsequent classes obtained in Mann–Whitney U test
For all water quality parameters, ZIPLAS differentiated between good and moderate classes, whereas in the best classes (high and good), differentiation was found for TP and SD. In the worst status, a clear overlap between poor and bad classes for all eutrophication indicators (TP, TN, SD) was noticed.
Discussion
Based on the physicochemical analysis, investigated lakes represented a varied spectrum of trophic conditions that are typical for Polish lakes (Siuda et al., 2013; Zdanowski, 1983). According to the WFD, the methods of assessing the ecological status should evaluate not only the quality of the water, but also the degree of deviation from conditions not disturbed by human activity. Among the analyzed lakes, six met the criteria of the reference lakes. This is crucial in understanding the role of zooplankton in assessing the ecological status of lakes, as so far, zooplankton has been used frequently in assessing trophic conditions and the difference between trophic and ecological status, which still remains unclear. Previous studies show that Rotifer indices are the best for the assessment of trophic status (Ejsmont-Karabin, 2012; Ferdous & Muktadir, 2009; Karabin, 1985). Both Crustacean and Rotifer indices were tested, since the literature also shows that Calanoida, which prefer low trophic conditions, may be good indicators of even a slight deterioration of the water quality in low-trophic lakes (Gannon & Stemberger, 1978).
For the development of the ZIPLAS multimetric index, metrics that correlated most strongly with all pressure indicators and reflected various aspects of the zooplankton community were selected. In total, five component metrics were selected as base for ZIPLAS multimetric development:
The percentage share of the Rotifer species indicative of high trophy in the indicative group’s number (IHTROT; %) showed the strongest correlation with the proxies of eutrophication. Indicator Rotifer species, typical for high-trophic lakes in Poland, are listed by Ganonn and Stemberger (1978) as indicator species of high-trophic lakes in North America. However, some morphological differences among species may exist even on the same continent. Therefore, using this index, region-specific list of indicator species for low and high trophy should be developed for different countries. Based on the research by Ejsmont-Karabin (2012) and Karabin (1985), the following species have been assigned as indicators of high trophy: Keratella cochlearis f. tecta, Keratella quadrata, Pompholyx sulcata, Filinia longiseta, Anuraeopsis fissa, Trichocerca pusilla, Brachionus angularis, and Brachionus diversicornis. Moreover, following were the indicators of low trophy: Ascomorpha ovalis, Conochilus hippocrepis, Ascomorpha ecaudis, Gastropus stylifer, and Polyarthra major.
The ratio of Calanoida to Cyclopoida individual numbers (CA/CY) was the only Crustacean index and the second strongest correlating index with TP. The value of this index decreases with increasing eutrophication. Based on the obtained results, it was found that the abundance of Calanoida decreases with an increase of trophy, while the abundance of Cyclopoida increases. This confirms the results of the research by Gannon and Stemberger, (1978), which showed that Calanoida prefers oligotrophic waters, where they are much more abundant, compared to waters of high trophy. These authors, based on the research of the American Great Lakes, concluded that Limnocalanus macrurus and Senecella calanoides belonging to the order Calanoida are effective indicators of low trophic waters, as they prefer cool, well-oxygenated waters.
Percentage of tecta form in the population of Keratella cochlearis (TECTA; %): Hillbricht-Ilkowska (1972) and Peljer (1962) show that in eutrophic lakes, Keratella cochlearis reaches a smaller body size and the length of their posterior spine is less than those inhabiting oligotrophic waters. The form without a posterior spine (tecta) is more common in eutrophic conditions. Obtained results agree with Ejsmont-Karabin (2012), showing that this indicator is not only one of the best indicators of trophic status but also an excellent indicator of the ecological status of stratified lakes.
Margalef’s index (d), which relates the number of species to the total number of individuals, was one of the two indicators of diversity tested in the study. In contrast to the Shannon Weaver index, it showed a highly statistically significant correlation with the parameters of pressure. To calculate Margalef’s index, the number of species and their abundance have to be taken into account, which means that the higher the index value, the better the ecological status of the lake. Obtained results show that an increase in the pollution level of the lake causes a decrease in the value of this index (see Fig. 2), which confirms the results of Haberman’s (1996, 1998) research, indicating that zooplankton species diversity decreases with increasing TP concentration.
Zooplankton abundance (NZOL; ind./L) is an indicator often used to assess the trophic status of lakes (Andronikova, 1996; Caroni & Irvine, 2010; Haberman & Haldna, 2014). It is well known that both the Rotifer and Crustacean abundance increase with an increase of trophy. The normalized values of NZOL index gradually decreased from 1 to 0.6 with increasing TP concentration, throughout the trophic gradient (see Fig. 2). This index is easy to calculate and is highly correlated with trophy (see Table 3).
The response of individual metrics to the increase in pressure expressed by TP concentration varied considerably (see Fig. 2). This was particularly evident in the case of the three metrics: CA/CY, IHTROT, and TECTA. CA/CY and IHTROT were most sensitive to a slight increase in TP concentration. The shape of the response curve of CA/CY metric is caused by displacement of Calanoida by Cyclopoida. Calanoida (mainly herbivores) occurs usually in oligotrophic environments where nanophytoplankton dominates (Hillbricht-Ilkowska, 1972). When conditions deteriorate, the domination is taken over by Cyclopoida, which prefers eutrophic conditions due to its ability to digest larger particles of food (Pace, 1986). IHTROT was also very sensitive to low TP concentrations (values decreased rapidly), while beyond 35 μgP/L of TP concentration, the decrease in the value became gradual. Due to the small body size of Rotifera, this group of zooplankton is released from the pressure of plankivorous fish, and thus, the population is regulated only by a bottom-up strategy (Ejsmont-Karabin, 2012). Changes in the community reflect a direct reaction to the enrichment of the waters with nutrients, and even a slight deterioration of the trophic conditions in reservoir causes a rapid increase in the share of species that prefer eutrophic conditions. Part of Rotifera preferring low-trophic waters are sensitive to the increase in eutrophication (similarly to Calanoida), and as the trophy increases, its abundance decreases. The reaction of this metric may result from the different sensitivities of individual indicator species to the trophic growth. Conversely, the TECTA metric was least sensitive to changes at low TP concentrations; however, its values decreased rapidly beyond 45 μg/L of TP concentration. The tecta form of Keratella cochlearis is absent in lakes of a very low trophy, while it is abundant in eutrophicated lakes (Ejsmont-Karabin, 2012). The morphological variability of Keratella cochlearis is probably determined by the abundance of phytoplankton and sestonu (Hillbricht-Ilkowska, 1972). This index is useful as a multimetric component for determining the border between good and moderate status, since tecta form does not occur in clean lakes (oligo- and mesotrophic). When the conditions in lakes deteriorate, the tecta form begins to appear in the zooplankton community and its abundance increases with an increase of trophy. The ZIPLAs multimetric index, with all of the above-mentioned components, enables the assessment of changes in the zooplankton community in the full trophic gradient.
ZIPLAS index values decrease with increasing lake eutrophication. This index is most sensitive to the deterioration of lake conditions, i.e., increase in TP and decline of SD; however, it is less sensitive in lakes where phosphorus values exceed 70 µg TP/L (see Fig. 3). The ZIPLAS differentiated between good and moderate status, which is crucial when developing biological methods in accordance with the WFD, indicating that this index is very sensitive to even a slight deterioration in lake water quality.
The zooplankton metrics presented above, which are components of the newly developed multimetric, are easy to calculate and do not require detailed knowledge of zooplankton species or the calculation of biomass according to complex formulas.
Additionally, one summer field campaign is sufficient to calculate a ZIPLAS multimetric. The summer stagnation is the most stable period, when changes in the abiotic and biotic environmental conditions are slight. During this period, zooplankton communities are most diversified and attain the highest abundance level (Karabin, 1985). Single sampling during the summer season is cost-efficient and has a potential to be useful for routine monitoring of lakes located in Poland and temperate zones. Moreover, the identification of zooplankton species is much easier than that of phytoplankton. Additionally, zooplankton samples are easy to collect and can be taken during the phytoplankton field campaign. All of these features make zooplankton a cost-efficient indicator that cannot be replaced by sampling fish or phytoplankton. Another potential consideration is the use of a newly developed zooplankton index to replace the costly monitoring of ichthyofauna, which not only interferes with the structure of the fish population but may also be inaccurate in the case of Polish lakes, where the assessment based on ichthyofauna remains debatable due to the continuous stocking processes. Mills et al. (1987) claim that zooplankton size can provide information regarding both the ratio of predator to prey and the structure of the fish community.
The proposed ZIPLAs multimetric index can be considered as a useful tool for assessing the ecological status of Polish lakes. It can also be used to assess lakes with similar abiotic types in temperature zone, while the use of the index in other regions requires adaptation of the list of indicator species.
Conclusions
Zooplankton is widely considered a central component of a pelagic food web in lakes. It is sandwiched between planktivorous fish (“top-down” control) and phytoplankton (“bottom-up” control), thus reflecting slight changes occurring in higher- and lower-trophic levels. As emphasized by Jeppesen et al. (2011), “Zooplankton has a strong indicator value, which cannot be covered by sampling fish and phytoplankton without a very comprehensive and costly effort.” (p. 279, abstact). The ZIPLAS serves as a new tool for measuring the ecological status of lakes and can provide a useful way to monitor even minor changes in lake water quality, derived from anthropogenic pressure. The sampling method used in the development of ZIPLAS is straightforward and cost-efficient compared to other biological methods and can be applied to other European stratified lakes in temperate zone. Results show that ZIPLAS would be a valuable addition to the WFD system, among the rest of the biological elements.
Availability of data and material
Data are available from the authors upon reasonable request.
References
Adamczyk, M., & Prus P. (2020). Ryby w jeziorach. In A. Kolada (Ed.), Podręcznik do monitoringu elementów 448 biologicznych i klasyfikacji stanu ekologicznego wód powierzchniowych. Environmental Monitoring Library, Warsaw. (in Polish) pp. 438
Andronikova, I. (1996). Zooplankton characteristics in monitoring of Lake Ladoga. Hydrobiologia, 322, 173–179. https://doi.org/10.1007/BF00031824
Arciszewski, M., Banaszak, K., Dondajewska, R., Gąbka, M., Gołdyn, R., Gołub, M., Heese, T., Hobot, A., Hutorowicz, A., Joniak, T., Kolada, A., Kołodziejczyk, A., Koperski, P., Kornijow, R., Korzec, E., Kostrzewska-Szlakowska, I., Kowalczewska-Madura, K., Kufel, L., Ławniczak, & Wojcieszonek, A. (2010). Sformułowanie w warunkach korzystania z wód regionu wodnego ograniczeń w korzystaniu z wód jezior lub zbiorników oraz w użytkowaniu ich zlewni. Praca wykonana w ramach Konsorcjum MGGP S.A. oraz Instytut Ochrony Środowiska na zlecenie KZGW, reprezentowanego przez Dyrektora Regionalnego Zarządu Gospodarki Wodnej w Krakowie. MGGP S.A. – IOŚ, Warsaw. http://www.rzgw.gda.pl/cms/site.files/file/RDW_2011/RZGW_Gdansk.pdf
Balushkina, E.V., & Vinberg, G.G. (1979). Dependences between mass and length of the body of plankton Crustacea. In N. Leningrad (Ed.), General basics of research in aquatic ecosystems (pp. 169–172). (in Russian)
Berzins, B., & Bertilsson, J. (1989). On limnic micro-crustaceans and trophic degree. Hydrobiologia, 185, 95–100. https://doi.org/10.1007/BF00010808
Berzins, B., & Pejler, B. (1989). Rotifer occurance and trophic degree. Hydrobiologia, 182, 171–180. https://doi.org/10.1007/BF00006043
Bielczyńska, A., Soszka H.& Tończyk G., (2020). Makrozoobentos w jeziorach. In A. Kolada (Ed.), Podręcznik do monitoringu elementów biologicznych i klasyfikacji stanu ekologicznego wód powierzchniowych. Environmental Monitoring Library, Warsaw. (in Polish) pp. 438
Birk, S., Bonne, W., Borja, A., Brucet, S., Courrat, A., Poikane, S., et al. (2012). Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators, 18, 31–41
Caroni, R., & Irvine, K. (2010). The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? Biology & Environment Proceedings of the Royal Irish Academy, 110B, 35–53
Carpenter, K. E., & J.M., & Buchanan, C. . (2006). An index of biotic integrity based on the summer polyhaline zooplankton community of the Chesapeake Bay. Marine Environmental Research, 62, 165–180. https://doi.org/10.1016/j.marenvres.2006.03.009
Čeirāns, A. (2007). Zooplankton indicators of trophy in Latvian lakes. Acta Universitatis Latviensis, 723, 61–69
Ciecierska, H., & Kolada, A. (2014). ESMI: A macrophyte index for assessing the ecological status of lakes. Environmental Monitoring and Assessment, 186, 5501–5517. https://doi.org/10.1007/s10661-014-3799-1
CIS (2003) Monitoring under the Water Framework Directive. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance Document No 7. Working Group 2.7 – Monitoring. European Communities, Luxembourg
Büttner, G., & Kosztra, B. (2017). CLC2018 Technical Guidelines. European Environment Agency, Wien. https://land.copernicus.eu/user-corner/technical-library/clc2018technicalguidelines_final.pdf (accessed in 10.01.2021)
De-Carli, B.P., Bressane, A., Longo, R.M., Manzi-Decarli, A., Moschini-Carlos, V., & Pompêo M.L.M. (2019). Development of a zooplankton biotic index for trophic state prediction in tropical reservoirs Limnetica. 38, 303–316. https://doi.org/10.23818/limn.38.21
Dembowska, E. A., Napiórkowski, P., Mieszczankin, T., & Józefowicz, Sz. (2015). Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecological Indicators, 56, 15–22 https://doi.org/10.1016/j.ecolind.2015.03.019
DeMott, W. R., & Kerfoot, W. C. (1982). Competition among cladocerans: Nature of the interactions between Bosmina and Daphnia. Ecology, 6, 1949–1666. https://doi.org/10.2307/1940132
EC (2000). Directive 2000/60 of the European Parliament and the Council of 23 October 2000 establishing a framework for community action
EEA (1996). EEA topic report 2: Inland waters: Surface water quality monitoring
Ejsmont-Karabin, J. (1998). Empirical equations for biomass calculation of planktonic rotifers. Polish Journal of Ecology, 45, 513–522
Ejsmont-Karabin, J. (2012). The usefulness of zooplankton as lake ecosystem indicators: Rotifer Trophic State Index. Polish Journal of Ecology, 60(2), 339–350
Ejsmont-Karabin, J., & Karabin, A. (2013). The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Polish Journal of Ecology, 61(3), 561–573
Organisation for Economic Cooperation and Development (OECD). (1982) Eutrophication of waters, monitoring, assessment, control. Paris, pp. 150
Ferdous, Z., & Muktadir, A. K. M. (2009). A review: Potentiality of zooplankton as bioindicator. American Journal of Applied Sciences, 6, 1815–1819. https://doi.org/10.3844/ajassp.2009.1815.1819
Gannon, J. E., & Stemberger, R. S. (1978). Zooplankton (especially crustacean and rotifers) as indicators of water quality. Transactions of the American Microscopical Society, 97, 16–35. https://doi.org/10.2307/3225681
Gliwicz, Z. M. (1969). The food sources of lake zooplankton. Ekologia Polska, 15, 205–223
Gulati, R. D. (1983). Zooplankton and its grazing as indicator of trophic status in Dutch lakes. Environmental Monitoring and Assessment, 3, 343–354
Haberman, J. (1996). Contemporary state of zooplankton in Lake Peipsi. Hydrobiologia, 338, 113–123. https://doi.org/10.1007/BF00031715
Haberman, J. (1998). Zooplankton of lake Võrtsjärv. Limnologica, 2, 49–65
Haberman, J., & Haldna, M. (2014). Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Vőrtsjärv. Journal of Limnology, 73(2), 263–273. https://doi.org/10.4081/jlimnol.2014.828
Hakkari, L. (1972). Zooplankton species as indicators of environment. Aqua Fennica, 1, 46–54
Hanson, J. M., & Peters, R. H. (1984). Empirical prediction of crustacean zooplankton biomass and profundal macrobenthos biomass in lakes. Canadian Journal of Fisheries and Aquatic Sciences, 41, 439–445. https://doi.org/10.1139/f84-052
Hering, D., Feld, C. K., Moog, O., & Ofenbock, T. (2006). Cook book for the development of a Multimetric Index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia, 566, 311–324. https://doi.org/10.1007/978-1-4020-5493-8_22
Hermanowicz, W., Dojlido, J., Dożańska, W., Koziorowski, B. & Zerbe, J. (1999). Physical-chemical examination of water and wastewater. Warszawa, Arkady (in Polish), pp. 556
Hillbricht-Ilkowska, A. (1972). Morphological variation of Keratella cochlearis (Gosse) (Rotatoria) in several Masurian lakes of different trophic level. Polskie Archiwum Hydrobiologii, 19, 253–264
Hutorowicz, A., & Pasztaleniec, A. (2014). Phytoplankton metric of ecological status assessment for Polish lakes and its performance along nutrient gradients. Polish Journal of Ecology, 62(3), 525–540. https://doi.org/10.3161/104.062.0312
Jeppesen, E., Jensen, J. P., Sondergaard, M., Lauridsen, T., & Landkildehus, F. (2000). Trophic structure, species richness and biodiversity in Danish lakes: Changes along nutrient and fish gradient. Journal of Plankton Research, 22, 951–968. https://doi.org/10.1046/j.1365-2427.2000.00675.x
Jeppesen, E., Noges, P., Davidson, T. A., Haberman, J., Noges, T., Blank, K., Lauridsen, T., Sondergaard, M., Sayer, C., Laugaste, R., Johansson, L. S., Bjerring, R., & Amsinck, S. L. (2011). Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia, 676, 279–297. https://doi.org/10.1007/s10750-011-0831-0
Kane, D., Gordon, S., Munawar, M., Charlton, M., & Culver, D. (2009). The planktonic index of biotic integrity (P-IBI): An approach for assessing lake ecosystem health. Ecological Indicators, 9, 1234–1247. https://doi.org/10.1016/j.ecolind.2009.03.014
Karabin, A. (1985). Pelagic zooplankton (Rotatoria + Crustacea) variation in the process of lake eutrophication. I. Structural and Quantitative Features. Ekologia Polska, 33, 567–616
Karpowicz, M., Sługocki, Ł, Kozłowska, J., Ochocka, A., & Lopez, C. (2020). Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecological Indicators, 117, 106585. https://doi.org/10.1016/j.ecolind.2020.106585
Kovach, W.L. (2007). MVSP—A MultiVariate Statistical Package for Windows, ver. 3.1. Kovach Computing Services, Pentraeth, Wales, U.K
Kutyła, S. (2020). The effect of hydromorphological pressures and catchment land use on the ecological status of the selected lakes in Poland. Dissertation, University of Life Sciences in Poznań. pp. 120
Lampert, W., & Sommer U. (2001). Ekologia wód śródladowych. Wydawnictwo Naukowe PWN. Warszawa
Lougheed, V. L., & Chow-Fraser, P. (2002). Development and use of a zooplankton index to monitor wetland quality in the Laurentian Great Lakes basin. Ecological Applications, 12(2), 474–486. https://doi.org/10.1890/1051-0761(2002)012[0474:DAUOAZ]2.0.CO;2
Lyche-Solheim, A. (ed.) (2005). Reference conditions of European lakes. Indicators and methods for the Water Framework Directive. Assessment of Reference conditions. − 105 pp., REBECCA project deliverable 7 http://www.rbm-toolbox.net/docstore/docs/3.1713.D7-uusi.pdf
Lyche-Solheim, A., Feld, Ch., Birk, S., Phillips, G., Carvalho, L., Morabito, G., Mischke, U., Willby, N., Sondergaard, M., Hellsten, S., Kolada, A., Mjelde, M., Böhmer, J., Pusch, M. T., Argillier, Ch., Jeppesen, E., Lauridsen, T. L., & Poikane, S. (2013). Ecological status assessment of European lakes: A comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia, 704, 57–74. https://doi.org/10.1007/s10750-012-1436-y
Margalef, D. R. (1958). Information Theory in Ecology. General Systems, 3, 36–71
Mills, E. L., Green, D. M., & Schiavone, A. (1987). Use of zooplankton size to assess the community structure of fish populations in freshwater lakes. North American Journal of Fisheries Management, 7, 369–378
Moss, B. (2007). Shallow lakes, the water framework directive and life. What should it all be about? Hydrobiologia, 584, 381–394. https://doi.org/10.1007/978-1-4020-6399-2_34
Nusch, E. A. (1980). Comparison of different methods for chlorophyll and pheopigment determination. Arch Hydrobiol Beih Ergebn Limnol, 14, 14–36
Ochocka, A., & Pasztaleniec, A. (2016). Sensitivity of plankton indices to lake trophic conditions. Environmental Monitoring and Assessment, 188(11), 622. https://doi.org/10.1007/s10661-016-5634-3
Pace, M. L. (1986). An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnology and Oceanography, 31, 45–55. https://doi.org/10.4319/lo.1986.31.1.0045
Pejler, B. (1962). On the variation of the rotifer Keratella cochlearis (Gosse). Zoologiska Bidrag Från Uppsala, 35, 307–319
Pejler, B. (1983). Zooplanktic indicators of trophy and their food. Hydrobiologia, 101, 111–114
Poikane, S., Salas, H. F., Kelly, M. G., Borja, A., Birk, S., & van de Bund, W. (2020). European aquatic ecological assessment methods: A critical review of their sensitivity to key pressures. Science of Total Environment, 740, 140075. https://doi.org/10.1016/j.scitotenv.2020.140075
Radwan, S., & Popiołek, B. (1989). Percentage of rotifers in spring zooplankton in lakes of different trophy. Hydrobiologia, 186(187), 235–238. https://doi.org/10.1007/978-94-009-0465-1_29
Shannon, C.E., & Weaver, W. (1963). The mathematical theory of communication. Urbana
Siuda, W., Kaliński, T., Kauppinen, E. & Chróst, R. (2013). Eutrophication of the southern part of the Great Masurian Lakes complex in the years 1977–2011. Causes, mechanisms and effects. [In Polish] Access protocol: http://www.zemuw.pl/pl/files/docs/JM_Eutrofizacja_jezior_mazurskich.pdf
Sládeček, V. (1983). Rotifers as indicators of water quality. Hydrobiologia, 100, 169–201. https://doi.org/10.1007/BF00027429
Sondergaard, M., Jeppesen, E., Jensen, J. P., & Amsinck, S. A. (2005). Water Framework Directive: Ecological classification of Danish lakes. Journal of Applied Ecology, 42, 616–629. https://doi.org/10.1111/j.1365-2664.2005.01040.x
Soszka, H. (2009). Methodical problems associated with the assessment of lake eutrophication for designation of zones vulnerable to nitrates. Water-Environment-Rural Areas, 9, 151–159
Soszka, H., Kolada, A., Gołub, M., & Cydzik, D. (2008). Establishing reference conditions for Polish lakes – preliminary results. Oceanological and Hydrobiological Studies, 37, 105–110. https://doi.org/10.2478/v10009-008-0010-4
Stamou, G., Katsiapi, M., Moustaka-Gouni, M., & Michaloudi, E. (2019). Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia, 844, 83–103. https://doi.org/10.1007/s10750-018-3880-9
StatSoft, Inc. (2014). STATISTICA (data analysis software system), version 12.0 www.statsoft.com
Zdanowski, B. (1983). Ecological characteristics of lakes in north-eastern Poland versus their trophic gradient. III. Chemistry of the water in 41 lakes. Ekologia Polska 31(2), 287–308
Zgrundo, A., Picińska–Fałtynowicz, J., & Pasztaleniec, A. (2020). Fitobentos w jeziorach. In A. Kolada (ed.) Podręcznik do monitoringu elementów biologicznych i klasyfikacji stanu ekologicznego wód powierzchniowych. Environmental Monitoring Library, Warsaw. (pp. 438) (in Polish)
Acknowledgements
The Chief Inspectorate for Environmental Protection in Poland is kindly acknowledged as the provider of the physico-chemical monitoring data used in this study.
Funding
This research was supported by the Polish National Science Centre by grant number 2012/07/N/NZ9/01396 and the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ochocka, A. ZIPLAS: Zooplankton Index for Polish Lakes’ Assessment: a new method to assess the ecological status of stratified lakes. Environ Monit Assess 193, 664 (2021). https://doi.org/10.1007/s10661-021-09390-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09390-7