Abstract
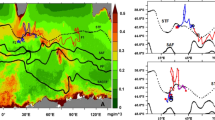
The spatial variation of chlorophyll a (Chl a) and factors influencing the high Chl a were studied during austral summer based on the physical and biogeochemical parameters collected near the coastal waters of Antarctica in 2010 and a zonal section along 60°S in 2011. In the coastal waters, high Chl a (>3 mg m−3) was observed near the upper layers (∼15 m) between 53°30′E and 54°30′E. A comparatively higher mesozooplankton biomass (53.33 ml 100 m−3) was also observed concordant with the elevated Chl a. Low saline water formed by melting of glacial ice and snow, as well as deep mixed-layer depth (60 m) due to strong wind (>11 ms−1) could be the dominant factors for this biological response. In the open ocean, moderately high surface Chl a was observed (>0.6 mg m−3) between 47°E and 50°E along with a Deep Chlorophyll Maximum of ∼1 mg m−3 present at 30–40 m depth. Melt water advected from the Antarctic continent could be the prime reason for this high Chl a. The mesozooplankton biomass (22.76 ml 100 m−3) observed in the open ocean was comparatively lower than that in the coastal waters. Physical factors such as melting, advection of melt water from Antarctic continent, water masses and wind-induced vertical mixing may be the possible reasons that led to the increase in phytoplankton biomass (Chl a).











Similar content being viewed by others
References
Ainley, D., & Jacobs, S. (1981). Sea-bird affinities for ocean and ice boundaries in the Antarctic. Deep-Sea Research Part I, 1(28), 1173–1185.
Anilkumar, N., Luis, A. J., Somayajulu, Y. K., Ramesh Babu, V., Dash, M. K., Pednekar, S. M., Babu, K. N., Sudhakar, M., & Pandey, P. C. (2006). Fronts, water masses and heat content variability in the western Indian sector of Southern Ocean during austral summer 2004. Journal of Marine Systems, 63, 20–34.
Assmy, P., Smetacek, V., Montresor, M., Klaas, C., Henjes, J., Strass, V. H., Arrieta, J. M., Bathmann, U., et al. (2013). Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic circumpolar current. Proceedings of the National Academy of Science. doi:10.1073/pnas.1309345110.
Banse, K. (1996). Low seasonality of low concentrations of surface chlorophyll in the sub Antarctic water ring: underwater irradiance, iron, or grazing? Progress in Oceanography, 37, 241–291.
Bathmann, U. V., Scharek, R., Klaas, C., Dubischar, C. D., & Smetacek, V. (1997). Spring development of phytoplankton biomass and composition in major water masses of the Atlantic sector of the Southern Ocean. Deep-Sea Research Part I, 44, 51–67.
Bianchi, F., Boldrin, A., Cioce, F., Dieckmann, G., Kuosa, H., Larsson, A. M., Nothig, E. M., Sehlstedt, P. I., Socal, G., & Syvertsen, E. E. (1992). Phytoplankton distribution in relation to sea ice, hydrography and nutrients in the north-western Weddell Sea in early spring 1988 during EPOS. Polar Biology, 12, 225–235.
Bindoff, N., Rosenberg, M., & Warner, M. (2000). On the circulation and water masses over the Antarctic continental slope and rise between 80°E and 150°E. Deep Sea Research, Part II, 47, 2299–2326.
Boyd, P. W., Watson, A. J., Law, C. S., Abraham, E. R., Trull, T., et al. (2000). A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature, 407, 695–702.
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., Coale, K. H., Cullen, J. J., debar, H. J. W., Follows, M., Harvey, M., Lancelot, C., Levasseur, M., Owens, N. P. J., Pollard, R., Rivkin, R. B., Sarmiento, J., Schoemann, V., Smetacek, V., Takeda, S., Tsuda, A., Turner, S., & Watson, A. J. (2007). Mesoscale iron enrichment experiments1993–2005: Synthesis and future directions. Nature, 315, 612–617.
Brandon, M. A., Naganobu, M., Deme, D. A., Chernyshkov, P., Trathan, P. N., Thorpe, S. E., Kameda, T., Berezhinskiy, O. A., Hawker, E. J., & Grant, S. (2004). Physical oceanography in the Scotia Sea during the CCAMLR 2000 survey, austral summer 2000. Deep-Sea Research Part II, 51, 1301–1321.
Calbet, A., Alcaraz, M., Atienza, D., Broglio, E., & Vaque, D. (2005). Zooplankton biomass distribution patterns along the western Antarctic Peninsula (December 2002). Journal of Plankton Research, 27, 1195–1203.
Cowles, T. J., & Fessenden, L. M. (1995). Copepod grazing and fine scale distribution patterns during the marine light-mixed layers experiment. Journal of Geophysical Research, 100, 6677–6686.
Dafner, E. V., & Mordasov, N. V. (1994). Influence of biotic factors on the hydrochemical structure of surface water in the polar frontal zone of the Atlantic Antarctic. Marine Chemistry, 45, 137–148.
De Baar, H. J. W., Buma, A. G. J., Nolting, R. F., Cadée, R. F., Jacques, G., & Tréguer, P. J. (1990). On iron limitation of the Southern Ocean: Experimental observations in the Weddell and Scotia seas. Marine Ecology Progress Series, 6, 105–122.
De Baar, H. J. W., de Jong, J. T. M., Bakker, D. C. E., Löscher, B. M., Veth, C., Bathmann, U. V., & Smetacek, V. (1995). Importance of iron for phytoplankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature, 373, 412–415.
Dierssen, H., & Smith, R. C. (2000). Bio-optical properties and remote sensing ocean color algorithms for Antarctic Peninsula waters. Journal of Geophysical Research, 105, 26301–26312.
Dierssen, H. M., Smith, R. C., & Vernet, M. (2002). Glacial meltwater dynamics in coastal waters west of the Antarctic Peninsula. Proceedings of the National Academy of Sciences, 99, 1790–1795.
Dong, S., Sprintall, J., Gille, S. T., & Talley, L. (2008). Southern Ocean mixed-layer depth from Argo float profiles. Journal of Geophysical Research, 113, C06013. doi:10.1029/2006JC004051.
Dugdale, R. C., & Wilkerson, F. P. (2001). Sources and fates of silicon in the ocean: The role of diatoms in the climate and glacial cycles. Scientia Marina, 65(Suppl 2), 141–152.
El-Sayed, S. (1994). Southern Ocean ecology: The BIOMASS perspective. CCAMLR science.
Estrada, M., & Delgado, M. (1990). Summer phytoplankton distributions in the Weddell Sea. Polar Biology, 10, 441–449.
Fonda Umani, S., Monti, M., Bergamasco, A., et al. (2005). Plankton community structure and dynamics versus physical structure from Terra Nova Bay to Ross Ice Shelf (Antarctica). Journal of Marine Systems, 55, 31–46.
Fragoso, G. M., & Smith, W. O., Jr. (2012). Influence of hydrography on phytoplankton distribution in the Amundsen and Ross Seas, Antarctica. Journal of Marine Systems, 89, 19–29.
Gandhi, N., Ramesh, R., Laskar, A. H., Sheshshayee, M. S., Shetye, S., Anilkumar, N., Patil, S. M., & Mohan, R. (2012). Zonal variability in primary production and nitrogen uptake rates in the southwestern Indian Ocean and the Southern Ocean. Deep-Sea Research Part I, 67, 32–43.
Garcı’a-Mun˜oz, C., Lubia’n, L. M., Carlos, M., Garcı’a, C. M., Marrero-Dı’az, A., Sangra, P., & Vernet, M. (2013). A mesoscale study of phytoplankton assemblages around the South Shetland Islands (Antarctica). Polar Biology, 36, 1107–1123.
Gloersen, P., Campbell, W. J., Cavalieri, D. J., Comiso, J. C., Parkinson, C. L., & Zwally, H. J. (1992). Arctic and Antarctic sea ice, 1978–1987: Satellite passive–microwave observations and analysis. Washington: National Aeronautics and Space Administration (NASA-SP511).
Goffart, A., Catalano, G., & Hecq, J. H. (2000). Factors controlling the distribution of diatoms and Phaeocystis in the Ross Sea. Journal of Marine Systems, 27, 161–175.
Goswami, S.C. (1983). Zooplankton of the Antarctica waters scientific report of first Indian expedition to Antarctica, technical publication 1.
Grasshoff, K. (1983). Methods of seawater analysis (eds Grasshoff, K., Ehrhardt, M. and Kremling, K.), Verlag Chemie, Weinheim, 2nd edn. p. 419.
Helbling, E. W., Villafan˜e, V., & Holm-Hansen, O. (1991). Effect of iron on productivity and size distribution of Antarctic phytoplankton. Limnology and Oceanography, 36, 1879–1885.
Hewitt, R. P., Demer, D. A., & Emery, J. H. (2003). An 8-year cycle in krill biomass density inferred from acoustic surveys conducted in the vicinity of the South Shetland Islands during the austral summers of 1991–1992 through 2001–2002. Aquatic Living Resources, 16, 205–213.
Heywood, K., Sparrow, M., Brown, J., & Dickson, R. (1999). Frontal structures and Antarctic bottom water flow through the princess Elizabeth trough, Antarctica. Deep-Sea Research Part I, 46(7), 1181–1200.
Hofmann, E. E., & Murphy, E. J. (2004). Advection, krill, and Antarctic marine ecosystems. Antarctic Science, 16, 487–499.
Holm-Hansen, O., Kahru, M., & Hewes, C. D. (2005). Deep chlorophyll a maxima (DCMs) in pelagic Antarctic waters. II. Relation to bathymetric features and dissolved iron concentrations. Marine Ecology Progress Series, 297, 71–81.
Hunt, B. P. V., & Hosie, G. W. (2005). Zonal structure of zooplankton communities in the Southern Ocean South of Australia: Results from a 2150 km continuous plankton recorder transect. Deep Sea Research, Part I, 52, 1241–1271.
Hutchins, D. A., Sedwick, P. N., DiTullio, G. R., Boyd, P. W., Queguiner, B., Griffiths, F. B., & Crossley, C. (2001). Control of phytoplankton growth by iron and silicic acid availability in the sub Antarctic Southern Ocean: Experimental results from the SAZ project. Journal of Geophysical Research, Oceans, 106, 31559–31572.
Jasmine, P., Muraleedharan, K. R., Madhu, N. V., Asha Devi, C. R., Alagarswamy, R., Achuthan Kutty, C. T., Jayan, Z., Sanjeevan, V. N., & Sahayak, S. (2009). Hydrographic and productivity characteristics along 45oE longitude in the southwestern Indian Ocean and Southern Ocean during Austral summer 2004. Marine Ecology Progress Series, 389, 97–116.
Kopczynska, E. E. (1988). Spatial structure of phytoplankton in the Scotia Front west Elephant Island (BIOMASSIII, October–November1986). Polish Polar Research, 9, 231–242.
Kopczynska, E. E., Dehairs, F., Elskens, M., & Wright, S. (2001). Phytoplankton variability between the subtropical and polar fronts south off Australia: Thriving under regenerative and new production in late summer. Journal of Geophysical Research, Oceans, 106, 31597–31609.
Laubscher, R. K., Perissinotto, R., & McQuaid, C. D. (1993). Phytoplankton production and biomass at frontal zones in the Atlantic sector of the Southern Ocean. Polar Biology, 13, 471–481.
Mackintosh, N. (1973). Distribution of post-larval krill in the Antarctic. Discovery Reports, 36, 1–94.
Martin, J. H. (1990). Glacial–interglacial CO2: The iron hypothesis. Paleoceanography, 5, 1–13.
Martin, J. H., Fitzwater, S. E., & Gordon, R. M. (1990). Iron deficiency limits phytoplankton growth in Antarctic waters. Global Biogeochemical Cycles, 4, 5–12.
Meijers, A. J. S., Klocker, A., Bindoff, N. L., Williams, G. D., & Marsland, S. J. (2010). The circulation and water masses of the Antarctic shelf and continental slope between 30 and 80°E. Deep-Sea Research Part II, 57, 723–737.
Mendes, C. R., de Souza, M. S., Garcia, V. M. T., Leal, M. C., Brotas, V., & Garcia, C. A. E. (2012). Dynamics of phytoplankton communities during late summer around the tip of the Antarctic Peninsula. Deep-Sea Research Part I, 65, 1–14.
Miller, D., & Monteiro, P. (1988). Antarctic Ocean and resources variability. Berlin: Springer.
Mitchell, B. G., & Holm-Hansen, O. (1991). Observations and modelling of the Antarctic phytoplankton crop in relation to mixing depth. Deep Sea Research, 38, 981–1007.
Moline, M., & Prezelin, B. B. (1996). Long-term monitoring and analyses of physical factors regulating variability in coastal Antarctic phytoplankton biomass, productivity and taxonomic composition over sub seasonal, seasonal and interannual time scales. Marine Ecology Progress Series, 145, 143–160.
Moline, M. A., Claustre, H., Frazer, T. K., Grzymski, J., Schofield, O., & Vernet, M. (2000). Antarctic ecosystems: Models for wider ecological understanding, eds. Davison, E., Howard-Williams, C. & Broady, P. (New Zealand Natural Sciences, Canterbury University, Christchurch, New Zealand).
Moore, J. K., & Abbott, M. R. (2002). Surface chlorophyll concentrations in relation to the Antarctic polar front: Seasonal and spatial patterns from satellite observations. Journal of Marine Systems, 37, 69–86.
Moore, J. K., Abbott, M. R., Richman, J. G., & Nelson, D. (2000). The Southern Ocean at the last glacial maximum: A strong sink for atmospheric carbon dioxide. Global Biogeochemical Cycles, 14, 455–475.
Morel, A., & Berthon, J. (1989). Surface pigments, algal biomass profiles, and potential production of the euphotic layer: Relationships reinvestigated in view of remote-sensing applications. Limnology and Oceanography, 34, 1545–1562.
Nicol, S., & Foster, J. (2000). Recent trends in the fishery for Antarctic krill. Aquatic Living Resources, 47, 2489–2517.
Nicol, S., Pauly, T., Bindoff, N., & Strutton, P. (2000). “BROKE” a biological/oceanographic survey off the coast of east Antarctica (80–150E) carried out in January–March 1996. Deep-Sea Research Part II, 47, 2281–2298.
Nicol, S., Meiners, K., & Raymond, B. (2010). BROKE-West, a large ecosystem survey of the South West Indian Ocean sector of the Southern Ocean (CCAMLR Division 58.4.2). Deep-Sea Research Part II, 57(9–10), 693–700.
Ohman, M. D., & Runge, J. A. (1994). Sustained fecundity when phytoplankton resources are in short supply: Omnivory by Calanus finmarchicus in the Gulf of St. Lawrence. Limnology and Oceanography, 39, 21–36.
Orsi, A. H., & Wiederwohl, C. L. (2009). A recount of Ross Sea waters. Deep Sea Research, Part II, 56, 778–795.
Orsi, A., Whitworth, T., III, Worth, D., & Nowlin, W., Jr. (1995). On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Research, Part I, 42, 641–673.
Parslow, J. S., Boyd, P. W., Rintoul, S. R., et al. (2001). A persistent subsurface chlorophyll maximum in the interpolar frontal zone south of Australia: Seasonal progression and implications for phytoplankton-light-nutrient interactions. Journal of Geophysical Research, 106, 31543–31557.
Pavithran, S., Anilkumar, N., Krishnan, K. P., Sharon, B. N., Jenson, V. G., Nanajkar, M., Chacko, R., Dessai, D. G. G., & Achuthankutty, C. T. (2012). Contrasting pattern in chlorophyll a distribution within the polar front of the Indian sector of Southern Ocean during austral summer 2010. Current Science, 102(6), 899.
Peloquin, J.A., & Smith Jr., W.O. (2007). Phytoplankton blooms in the Ross Sea, Antarctica: Interannual variability in magnitude, temporal patterns, and composition. Journal of Geophysical Research-Oceans, 112, doi: 10.1029/2006JC003816.
Sangrà, P., García-Muñoz, C., García, C. M., Marrero-Díaz, A., Sobrino, C., Mouriño-Carballido, B., Aguiar-González, B., Henríquez-Pastene, C., Rodríguez-Santana, A., Lubián, L. M., Hernández-Arencibia, M., Hernández-León, S., Vázquez, E., & Estrada-Allis, S. N. (2014). Coupling between upper ocean layer variability and size-fractionated phytoplankton in a non-nutrient-limited environment. Marine Ecology Progress Series, 499, 35–46. doi:10.3354/meps10668.
Sarmiento, J. L., Hughes, T. M. C., Stouffer, R. J., & Manabe, S. (1998). Simulated response of the ocean carbon cycle to anthropogenic climate warming. Nature, 393(6682), 245–249.
Sedwick, P. N., Edwards, P. R., Mackey, D. J., Griffiths, F. B., & Parslow, J. S. (1997). Iron and manganese in surface waters of the Australian sub Antarctic region. Deep-Sea Research Part I, 44, 1239–1253.
Sedwick, P. N., DiTullio, G. R., Hutchins, D. A., Boyd, P. W., Griffiths, F. B., Crossley, A. C., Trull, T. W., & Que’guiner, B. (1999). Limitation of algal growth by iron deficiency in the Australian Subantarctic region. Geophysical Research Letters, 26, 2865–2868.
Smith, W. O., Jr., & Nelson, D. M. (1986). Importance of ice edge phytoplankton production in the Southern Ocean. Bioscience, 36, 251–257.
Smith, W. O., Ainley, D. G., & Catteneo-Vietti, R. (2007). Trophic interactions within the Ross Sea continental shelf ecosystem. Philosophical Transactions of the Royal Society B, 362, 95–111.
Sokolov, S. (2008). Chlorophyll blooms in the Antarctic Zone south of Australia and New Zealand in reference to the Antarctic circumpolar current fronts and sea ice forcing. Journal of Geophysical Research Oceans, 113, C03022. doi:10.1029/2007JC004329.
Sokolov, S., & Rintoul, S. R. (2007). Multiple jets of the Antarctic circumpolar current south of Australia. Journal of Physical Oceanography, 37, 1394–1412.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis. Journal of the Fisheries Research Board of Canada, 167, 310.
Strutton, P., Griffiths, B., Waters, R., Wright, S., & Bindoff, N. L. (2000). Primary productivity off the coast of East Antarctica (80 to 150°E): January to March 1996. Deep-Sea Research Part II, 47, 2327–2363.
Swadling, K. M., Kowaguchi, S., & Hosie, G. W. (2010). Antarctic mesozooplankton community structure during BROKE-WEST (301E-801E), January–February 2006. Deep Sea Research, Part II, 57, 887–904.
Takahashi, T., Sutherland, S. C., Sweeney, C., Poisson, A., Metzl, N., Tilbrook, B., Bates, N., Wanninkhof, R., Feely, R. A., Sabine, C., Olafsson, J., & Nojiri, Y. (2002). Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effect. Deep Sea Research, Part II, 49, 1601–1622.
Uno, S. (1982). Distribution and stocking stock of chlorophyll a in the Antarctic Ocean, from December 1980 to January1981. Memoirs of the National Institute for Polar Research (Special Issue), 23, 20–27.
Van Leeuwe, M. A., Scharek, R., de Baar, H. J. W., de Jong, J. T. M., & Goeyens, L. (1997). Iron enrichment experiments in the Southern Ocean: Physiological responses of plankton communities. Deep Sea Research, Part II, 44, 189–208.
Westwood, K., Griffiths, F., Meiners, K., & Williams, G. (2010). Primary productivity off the Antarctic coast from 30° to 80°E; BROKE-West survey, 2006. Deep Sea Research, Part II, 57(9–10), 794–814.
Whitworth, T., III, Orsi, A., Kim, S.-J., & Nowlin, W., Jr. (1998). Water masses and mixing near the Antarctic slope front. In S. Jacobs & R. Weiss (Eds.), Ocean, ice and atmosphere: Interactions at the Antarctic continental margin (pp. 1–27). Washington: American Geophysical Union.
Williams, G. D., Nicol, S., Raymond, B., & Meiners, K. (2008). On the summer time mixed layer development in the marginal sea-ice zone off the Mawson coast, east Antarctica. Deep-Sea Research Part II, 55, 365–376.
Williams, G. D., Nicol, S., Aoki, S., Meijers, A. J. S., Bindoff, N. L., Iijima, Y., Marsland, S. J., & Klocker, A. (2010). Surface oceanography of BROKE-West, along the Antarctic margin of the south-west Indian Ocean (30 to 80°E). Deep-Sea Research Part II, 57, 738–757.
Wright, S. W., & van den Enden, R. L. (2000). Phytoplankton community structure and stocks in the East Antarctic marginal ice zone (BROKE survey, Jan–Mar. 1996) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Research, Part II, 47, 2363–2400.
Wright, S. W., van den Enden, R. L., Pearce, I., Davidson, A. T., & Scott, F. (2010). Phytoplankton community structure and stocks in the Southern Ocean (30–801E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Research, Part II, 57(9–10), 758–778.
Acknowledgements
This work was supported by the Ministry of Earth Sciences, Government of India. We are thankful to Director, NCAOR, for his constant support and encouragement. We are indebted to Ms. Usha Parameshwaran and Dr. Abdul Jaleel, CMLRE, Kochi, for the statistical analysis. The authors acknowledge the contribution towards data collection and analysis carried out by Dr. Sini Pavithran, Dr. Mandar Nanajkar, Dr. Deepti Dessai and Ms. Sharon B. Noronha. Help rendered in the implementation and completion of this study by cruise participants and staff at NCAOR is acknowledged. The authors are grateful to the anonymous reviewer for the valuable suggestions. This is NCAOR contribution no 18/2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anilkumar, N., Chacko, R., Sabu, P. et al. Biological response to physical processes in the Indian Ocean sector of the Southern Ocean: a case study in the coastal and oceanic waters. Environ Monit Assess 186, 8109–8124 (2014). https://doi.org/10.1007/s10661-014-3990-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3990-4