Abstract
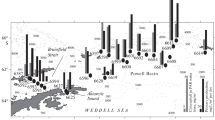
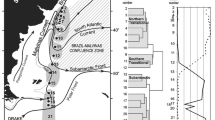
A high resolution study of chlorophyll a and primary production distribution was carried out in the Atlantic sector of the Southern Ocean during the austral summer of 1990–91. Primary production (14C assimilation) and photosynthetic capacity levels at frontal systems were among the highest recorded during the cruise (2.8–6.3 mgC·m−3·h−1, and 1.3–4.7mgC·mgChl a −1·h−1, respectively). Blooms at ocean fronts were strongly dominated by specific size classes and species. This suggests that the increase in biomass was probably the result of an enhancement of in situ production by selected components of the phytoplankton assemblage, rather than accumulation of cells through hydrographic forces. This hypothesis is supported by the high variability of photosynthetic capacities at adjacent stations along the transects. Blooms (ca 2.7–3.5 mg Chl a·m−3) were found at three oceanic fronts (the Subtropical, Subantarctic and Antarctic Polar Fronts) during the early summer. These were equivalent to, or denser than, blooms in the Marginal Ice Zone and at the Continental Water Boundary. Seasonal effects on phytoplankton community structure were very marked. In early summer (December), netphyto-plankton (>20 μm) was consistently the major component of the frontal blooms, with the chain-forming diatoms Chaetoceros spp. and Nitzschia spp. dominating at the Subantarctic and Antarctic Polar Fronts, respectively. During late summer (February), nanophytoplankton (1–20 μm) usually dominated algal communities at the main frontal areas. Only at the Antarctic Polar Front did netphytoplankton dominate, with the diatom component consisting almost exclusively of Corethron criophilum. An early to late summer shift of maximum phytoplankton biomass from north to south of the Antarctic Polar Front was observed. Spatial covariance between silicate levels and water-column stability appeared to be the main factor controlling phytoplankton production at the Antarctic Polar Front. Low silicate concentrations may have limited diatom growth at the northern edge of the front, while a deep mixed layer depth reduced production at the southern edge of the front.
Similar content being viewed by others
References
Ainly DG, Jacobs SS (1981) Sea-bird affinities for ocean and ice boundaries in the Antarctic. Deep-Sea Res 10:1173–1185
Allanson BR, Hart RC, Lutjeharms JRE (1981) Observations on the nutrients, chlorophyll and primary production of the Southern Ocean south of Africa. S Afr J Ant Res 10/11:3–13
Allanson BR, Boden BP, Parker L, Duncombe Rae C (1985) A contribution to the oceanology of the Prince Edward Islands. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin Heidelberg New York, pp 38–45
Berman T, Kimor B (1983) A large-scale filtration apparatus for net plankton sampling. J Plankton Res 5:111–116
Bidigare RR, Frank TJ, Zastrow C, Brooks JM (1986) The distribution of algal chlorophylls and their degradation products in the Southern Ocean. Deep-Sea Res 33 (7):923–937
Crawford AB (1972) Sea surface temperature measurement by direct sampling. Maritime Weather Office, Cape Town, 8 pp
Deacon GER (1982) Physical and biological zonation in the Southern Ocean. Deep-Sea Res 29 (1A):1–15
El-Sayed S (1982) Spatial and temporal variation in phytoplankton biomass and primary productivity in the southwest Atlantic and the Scotia Sea. Polar Biol 1:83–90
El-Sayed S (1984) Productivity of the Antarctic waters-A reappraisal. In: Holm-Hansen O, Bolis L, Gilles R (eds) Marine Phytoplankton and Productivity. Springer, Berlin Heidelberg New York, pp 19–34
Eppley RW (1972) Temperature and phytoplankton growth in the sea. Fish Bull 70 (4):1063–1085
Franks PJS (1992) Sink or swim: accumulation of biomass at fronts. Mar Ecol Prog Ser 82:1–12
Fukuchi M (1980) Phytoplankton chlorophyll stocks in the Antarctic Ocean. J Oceanogr Soc Japan 36 (1):73–84
Fukuchi M, Tamura S (1982) Chlorophyll a distribution in the Indian sector of the Antarctic Ocean in 1978–1979. Antarctic Rec 74:143–162
Goeyens L, Sorensson F, Treguer P, Morvan J, Panouse M, Dehairs F (1991a) Spatiotemporal variability of inorganic nitrogen stocks and uptake fluxes in the Scotia-Weddell Confluence area during November and December 1988. Mar Ecol Prog Ser 77:7–19
Goeyens L, Treguer P, Lancelot C, Mathot S, Becquevort S, Morvan, J, Dehairs F, Baeyens W (1991b) Ammonium regeneration in the Scotia-Weddell Confluence area during spring 1988. Mar Ecol Prog Ser 78:241–252
Hart TJ (1934) On the phytoplankton of the south-west Atlantic and the Bellinghausen Sea, 1929–1931. ‘Discovery’ Rep 8, pp 1–268
Hart TJ (1942) Phytoplankton periodicity in Antarctic surface waters. ‘Discovery’ Rep 21, pp 261–356
Hasle GR (1978) The inverted microscope method. In: Sournia A (ed) Monographs on oceanographic methodology, Vol 6, phytoplankton manual. UNESCO, Paris, pp 88–96
Hayes PK, Whitaker TM, Fogg GE (1984) The distribution and nutrient status of phytoplankton in the Southern Ocean between 20° and 70°W. Polar Biol 3:153–165
Heywood RB, Priddle J (1987) Retention of phytoplankton by an eddy. Cont Shelf Res 7 (8):937–955
Holm-Hansen O, El-Sayed SZ, Franceschini GA, Cuhel RL (1977) Primary production and the factors controlling phytoplankton growth in the Southern Ocean. Proceedings of the Third SCAR Symposium on Antarctic biology. Adaptations within Antarctic ecosystems. Gulf Publishing Company, Houston, pp 11–50
Jacques G (1983) Some ecophysiological aspects of the Antarctic phytoplankton. Polar Biol 2:27–33
Jacques G (1989) Primary production in the open Antarctic Ocean during the austral summer. A review. Vie Milieu 39 (1):1–17
Jennings JC Jr, Gordon LI, Nelson DM (1984) Nutrient depletion indicates high primary productivity in the Weddell Sea. Nature. 309:51–54
Jones EP, Nelson DM, Treguer P (1990) Chemical Oceanography In: Smith WO (ed) Polar Oceanography Part B: Chemistry, Biology, and Geology. Academic Press, San Diego, pp 407–476
Kanda H, Fukuchi M (1979) Surface chlorophyll a concentration along the course of the Fuji to and from Antarctica in 1977–1978. Antarctic Rec 66:37–49
Koroleff F (1983) Determination of ammonia and urea. In: Grasshof K, Gerhardt M and Kremling K (eds) Methods of sea-water analysis. Verlag Chemie, Weinheim, pp 50–162
Lancelot C, Veth C, Mathot S (1991) Modelling ice-edge phytoplankton bloom in the Scotia-Weddell sea sector of the Southern Ocean during spring 1988. J Mar Syst 2:333–346
Legendre L (1985) Hydrodynamic control of marine phytoplankton production: The paradox of stability. In: Nihoul JCJ (ed) Marine Interfaces Ecohydrodynamics. Elsevier Oceanography Series, pp 191–207
Lewis MR, Smith JC (1983) A small volume short-incubation time method for measurement of photosynthesis as a function of incident irradiance. Mar Ecol Prog Ser 13:99–102
Lutjeharms JRE, Foldvik A (1986) The thermal structure of the upper ocean layers between Africa and Antarctica during the period December 1978 to March 1979. S Afr J Antarct Res 16 (1): 13–20
Lutjeharms JRE Valentine, HR (1984) Southern Ocean thermal fronts south of Africa. Deep-Sea Res 31 (12):1461–1475
Lutjeharms JRE, Walters NM, Allanson BR (1985) Oceanic frontal systems and biological enhancement. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic Nutrient Cycles and Food Webs. Springer, Berlin Heidelberg New York pp 11–21
Lutjeharms JRE, Allanson BR, Parker L (1986) Frontal zones, chlorophyll and primary production patterns in the surface waters of the Southern Ocean south of Cape Town. In: Nihoul JCJ (ed) Marine Interfaces Ecohydrodynamics. Elsevier Oceanography Series, pp 105–117
Marra J, Boardman DC (1984) Late winter chlorophyll a distribution in the Weddell Sea. Mar Ecol Prog Ser 19:197–205
Martin JH (1990) Glacial-interglacial CO2 change: the iron hypothesis. Palaeoceanography 5:1–13
Martin JH, Fitzwater SE (1988) Iron deficiency limits phytoplankton growth in the north-east Pacific Subarctic. Nature 331:341–343
Martin JH, Gordon RM, Fitzwater SE (1990) Iron in Antarctic waters. Nature 345:156–158
Mitchell BG, Holm-Hansen O (1991) Observations and modelling of the Antarctic phytoplankton crop in relation to mixing depth. Deep Sea Res 38:981–1007
Mordasova NV (1989) Chlorophyll distribution in the Antarctic zone of the Atlantic Ocean. Oceanol 29 (3):368–374
Mostert SA (1983) Procedures used in South Africa for the automatic photometric determinaton of micro-nutrients in sea-water. S Afr J Mar Sci 1:189–198
Oison RJ (1980) Nitrate and ammonia uptake in Antarctic waters. Limnol Oceanogr 25 (6):1064–1074
Olson DB, Backus RH (1985) The concentration of organisms at fronts: A cold-water fish and a warm-core Gulf Stream Ring. J Mar Res 43:113–137
Palmisano AC, SooHoo JB, Sullivan CW (1987) Effects of four environmental variables on photosynthesis-irradiance relationships in Antarctic sea-ice microalgae. Mar Biol 94:299–306
Perissinotto R, Duncombe Rae CM, Boden BP, Allanson BR, (1990) Vertical stability as a controlling factor of the marine phytoplankton production at the Prince Edward Archipelago (Southern Ocean). Mar Ecol Prog Ser 60:205–209
Perissinotto R, Laubscher RK, McQuaid CD (1992) Marine productivity enhancement around Bouvet and the South Sandwich Islands (Southern Ocean). Mar Ecol Prog Ser 88:41–53
Peterson RG, Stramma L (1991) Upper-level circulation in the South Atlantic Ocean. Prog Oceanog 26:1–73
Platt T Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38(4):687–701
Priddle J, Hawes I, Ellis-Evans JC (1986a) Antarctic aquatic ecosystems as habitats for phytoplankton. Biol Rev 61:199–238
Priddle J, Heywood RB, Theriot E (1986b) Some environmental factors influencing phytoplankton in the Southern Ocean around South Georgia. Polar Biol 5:65–79
Probyn TA (1988) Nitrogen utilization by the phytoplankton in the Namibian upwelling region during an austral spring. Deep-Sea Res 35 (8):1387–1404
Probyn TA, Painting SJ (1985) Nitrogen uptake by size-fractionated phytoplankton populations in Antarctic surface waters. Limnol Oceanogr 30:1327–1332
Sakshaug E, Holm-Hansen O (1984) Factors governing pelagic production in polar oceans. In: Holm-Hansen O, Bolis L and Gilles R (eds) Marine Phytoplankton and Productivity. Springer, Berlin Heidelberg New York, pp 19–34
Sasaki H (1984) Distribution of nano- and microplankton in the Indian sector of the Southern Ocean. Mem Natl Inst Polar Res, Spec Issue, 32:38–50
Smith WO (1987) Phytoplankton dynamics in marginal ice zones. Oceanogr Mar Biol Annual Rev 25:11–38
Smith WO, Harrison WG (1991) New production in polar regions: the role of environmental controls. Deep-Sea Res 38 (12):1463–1479
Smith WO, Nelson DM (1985) Phytoplankton bloom produced by a receding ice edge in the Ross Sea: Spatial coherence with a density field. Science 227:163–166
Smith WO, Nelson DM (1986) Importance of ice edge phytoplankton production in the Southern Ocean. BioScience 36 (4):251–257
Smith WO, Keene NK, Comiso JC (1988) Interannual variability in estimated productivity of the Antarctic marginal ice zone. In: Sahrhage D (ed) Antarctic Ocean and Resources Variability. Springer, Berlin Heidelberg New York, pp 131–139
Strickland JDH, Parsons TR (1968) A manual of sea-water analysis. Fisheries Research Board of Canada, Ottawa, 302 pp
Tanimura A (1981) Distribution of the surface chlorophyll a along the course of the Fuji to and from Antarctica in 1979–1980. Antarctic Rec 72:35–48
Tilzer MM, Dubinsky Z (1987) Effects of temperature and daylength on the mass balance of Antarctic phytoplankton. Polar Biol 7:35–42
Tilzer MM, Elbrächter M, Gieskes WW, Beese B (1986) Light-temperature interactions in the control of photosynthesis in Antarctic phytoplankton. Polar Biol 5:105–111
Tilzer MM, Bodungen B von, Smetacek V (1985) Light-dependance of phytoplankton photosynthesis in the Antarctic Ocean: Implications for regulating productivity. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic Nutrient Cycles and Food Webs. Springer, Berlin Heidelberg New York, pp 60–69
Verlencar XN, Somasunder K, Qasim SZ (1990) Regeneration of nutrients and biological productivity in Antarctic waters. Mar Ecol Prog Ser 61:41–59
Voronina NM (1962) On the dependance of the character of the boundary between Antarctic and Sub-Antarctic pelagic zones on the meteorological conditions. Antarctic Res Maury Memor Symp, Am Geophys Union, pp 160–162
Watanabe K, Nakajima Y (1983) Surface distribution of chlorophyll-a along the course of the Fuji (1980/81) in the Southern Ocean. Antarct Rec 77:33–43
Weber LH, El Sayed S (1987) Contributions of the net, nano- and picoplankton to the phytoplankton standing crop and primary productivity in the Southern Ocean. J Plank Res 9 (5):973–994
Wyrtki K (1960) The Antarctic Convergance — and Divergance Nature 187:581–582
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laubscher, R.K., Perissinotto, R. & McQuaid, C.D. Phytoplankton production and biomass at frontal zones in the Atlantic sector of the Southern Ocean. Polar Biol 13, 471–481 (1993). https://doi.org/10.1007/BF00233138
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00233138