Abstract
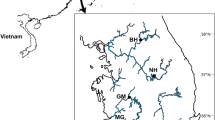
Neanthes glandicincta (Nereididae, Polychaeta) is the first numerically dominant benthic infauna in the Mai Po international Ramsar site, Hong Kong and also an economically important species for food source of birds and fishes. In present study, highly conserved nuclear ribosomal DNA (SSU and LSU rDNA) and mitochondrial COI gene were employed to study the population structure of N. glandicincta in the subtropical mudflat. The specimens were collected from five localities in February 2006, February–August 2007 and preserved at −80 °C, methanol or formalin, respectively. DNA extraction efficiency was the highest in fresh materials and lowest in formalin-fixed samples. The 18S (1774 bp), 28S D1 (383 bp) and COI genes were sequenced and analyzed. Both 18S and 28S D1 rDNA were highly conserved and showed no difference among the populations, whereas COI gene exhibited relatively high-level intraspecific polymorphism (2.2 %). The population from onshore and near mangrove station was phylogenetic different from other sites, indicating restricted gene exchange between the region of river mouth and mangrove forest. The mangrove may form a barrier for the dispersal of pelagic/benthic larvae of the population, which indicates that the population genetic difference is related to different habitats.




Similar content being viewed by others
References
Bertozzini E, Penna A, Bruce I, Magnani M (2005) Development of new procedures for the isolation of phytoplankton DNA from fixed samples. J Appl Phycol 17:223–229
Black MB, Halanych KM, Maas PAY, Hoeh WR, Hashimoto J, Desbruyères D, Lutz RA, Vrijenhoek RC (1997) Molecular systematics of vestimentiferan tubeworms from hydrothermal vents and cold-water seeps. Mar Biol 130:141–149
Bleidorn C (2005) Phylogenetic relationships and evolution of Orbiniidae (Annelida, Polychaeta) based on molecular data. Zool J Linn Soc 144:59–73
Brown WM, George M Jr, Wilson AC (1979) Rapid evolution of mitochondrial DNA. Proc Natl Acad Sci USA 76:1967–1971
Brusca RC, Brusca GJ (2003) Invertebrates, 2nd edn. Sinauer Associates Inc, Sunderland
Bryan GW, Hummerstone LG (1971) Adaptation of the polychaete Nereis diversicolor to estuarine sediments containing high concentrations of heavy metals. J Mar Biol Assoc UK 51:845–863
Fauchald K (1977) The polychaete worms. Definitions and keys to the orders, families and genera. Nat Hist Mus Los Angel County Sci Ser 28:1–188
Gambi MC, Castelli A, Giangrande A, Lanera P, Predevelli D, Vandini RZ (1994) Polychaetes of commercial and applied interest in Italy: an overview. Mem Mus Natl Hist Nat 162:593–603
Gooch L (1975) Mechanisms of evolution and population genetics, Vol 2 (1). In: Kinne O (ed) Marine ecology: a comprehensive, integrated treatise on life in oceans and coastal waters. Wiley, New York, pp 351–409
Greer CE, Peterson SL, Kiviat NB, Manos MM (1991) PCR amplification from paraffin embedded tissues. Am J Clin Pathol 95:117–124
Halanych KM, Janosik AM (2006) A review of molecular markers used for Annelid phylogenetics. Integr Comp Biol 46:533–543
Hart MW, Byrne M, Smith MJ (1997) Molecular phylogenetic analysis of life-history evolution in asterinid starfish. Evolution 51:1848–1861
Hateley JG, Grant A, Jones NV (1989) Heavy metal tolerance in estuarine populations of Nereis diversicolor. In: Ryland JS, Tyler PA (eds) Reproduction, genetics and distributions of marine organisms. Proceedings of the 23rd European Marine Biology Symposium, School of Biological Sciences, University of Wales, Swansea, Fredensborg, Denmark, pp. 379–385
Hebert PDN, Cywinska A, Ball SL, de Waard JR (2003) Biological identifications through DNA barcodes. P R Soc B 270:313–321
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453
Hills P, Zhang L, Liu J (1998) Trans boundary pollution between Guangdong Province and Hong Kong: threats to water quality in the Pearl River estuary and their implications for environmental policy and planning. J Environ Planning Manag 41:375–396
Hurtado LA, Lutz RA, Vrijenboek RC (2004) Distinct patterns of genetic differentiation among annelids of eastern Pacific hydrothermal vents. Mol Ecol 13:2603–2615
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Mol Evol 16:111–120
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol S 24:189–216
Knowlton N (2000) Molecular genetic analyses of species boundaries in the sea. Hydrobiologia 420:73–90
Kojima S (1998) Paraphyletic status of Polychaeta suggested by phylogenetic analysis based on the amino acid sequences of elongation factor-1-alpha. Mol Phylogenet Evol 9:255–261
Kojima S, Ohta S, Yamamotot T, Yamaguichi T, Miura T, Fujiwara Y, Fuijikura K, Hashimoto J (2003) Molecular taxonomy of vestimentiferans of the western Pacific, and their phylogenetic relationship to species of the eastern Pacific III. Alasia-like vestimentiferans and relationships among families. Mar Biol 142:625–635
Kruse I, Reusch TBH, Schneider MV (2003) Sibling species or poecilogony in the polychaete Scoloplos armiger? Mar Biol 142:937–947
Lai MY (2004) Fractionation, mobilization and bioaccumulation of heavy metals and mineralogical characteristics of the Mai Po Inner Deep Bay mudflat. M. Phil. thesis, The University of Hong Kong
Li B, Bisgaard HC, Forbes VE (2004) Identification and expression of two novel cytochrome P450 genes, belonging to CYP4 and a new CYP331 family, in the polychaete Capitella capitata sp.I. Biochem Biophys Res Commun 325:510–517
Lunt DH, Zhang DX, Szymura JM (1996) The insect cytochrome oxidaseIgene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol 5:153–165
McHugh D (1997) Molecular evidence that echiurans and pogonophorans are derived annelids. Proc Natl Acad Sci USA 94:8006–8009
McHugh D (2000) Molecular phylogeny of Annelida. Can J Zool 78:1873–1884
McHugh D (2001) Molecular phylogenetic analyses indicate a rapid radiation of polychaete annelids. Am Zool 41:1520–1521
McHugh D (2005) Molecular systematics of polychaetes (Annelida). Hydrobiologia 535:309–318
Moriyama EN, Powell JR (1997) Synonymous substitutions rates in Drosophila: mitochondrial versus nuclear genes. J Mol Evol 45:378–391
Neller RJ, Lam KC (1994) The environment. In: Yeung YM, Chu DKY (eds) Guangdong: survey of a province undergoing rapid change. The Chinese University Press, Hong Kong, pp 401–428
Nygren A, Sundberg P (2003) Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Mol Phylogenet Evol 29:235–249
Palumbi SR, Cipriano F, Hare MP (2001) Predicting nuclear gene coalescence from mitochondrial data: the three-times rule. Evolution 55:859–868
Passamaneck Y, Halanych KM (2006) Lophotrochozoan phylogeny assessed with LSU and SSU data: evidence of lophophorate polyphyly. Mol Phyl Evol 20:20–28
Polanco C, González AI, Dover GA (2000) Patterns of variation in the intergenic spacers of ribosomal dna in drosophila melanogaster support a model for genetic exchanges during X-Y pairing. Genetics 155:1221–1229
Reisch DJ (1984) Marine ecotoxicological tests with polychaetous annelids. In: Persoone G, Jaspers E, Claus C (eds). Ecotoxicological testing for the marine environment. vol 1, pp 427–454. State University of Ghent and Institute of Marine Scientific Research, Ghent
Remigio EA, Hebert PDN (2003) Testing the utility of partial COI sequences for phylogenetic estimates of gastropod relationships. Mol Phyl Evol 29:641–647
Rousset V, Rouse GW, Feral JP, Desbruyeres D, Pleijel F (2003) Molecular and morphological evidence of Alvinellidae relationships (Terebelliformia, Polychaeta, Annelida). Zool Scripta 32:185–197
Rousset V, Rouse G, Siddall ME, Tillier A, Pleijel F (2004) The phylogenetic position of Sibolginidae (Annelida) inferred from 18S rRNA, 28S rRNA and morphological data. Cladistics 20:518–533
Shen PP, Zhou H, Lai HY, Gu JD (2006) Benthic infaunal composition and distribution at an intertidal wetland mudflat. Water Air Soil Pollut 6:575–581
Shen PP, Zhou H, Lai HY, Gu JD (2010) Patterns of polychaete communities in relation to environmental perturbations in a subtropical wetland of Hong Kong. J Mar Biol Assoc UK 90:923–932
Shen PP, Zhou H, Gu JG (2012) Novel polymorphism of internal transcribed spacers (ITS) and their utilization in phylogenetic analysis of Neanthes glandicincta (Annelida: Polychaeta: Nereididae). Ecotoxicology 21:1717–1725
Shi SR, Cote RJ, Wu L, Liu C, Datar R, Shi Y, Liu DX, Lim H, Taylor CR (2002) DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. J Histochem Cytochem 50:1005–1011
Shin PKS (2001) Population Dynamics and secondary production of Neanthes glandicincta (Polychaeta: Nereididae) from a subtropical mudflat. Asian Mar Biol 18:117–127
Shin PKS, Ellingsen KE (2004) Spatial patterns of soft-sediment benthic diversity in subtropical Hong Kong waters. Mar Ecol Prog Ser 276:25–35
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Weinberg JR, Starczak VR, Mueller C, Pesch GC, Lindsay SM (1990) Divergence between populations of a monogamous polychaete with male parental care: premating isolation and chromosome variation. Mar Biol 107:205–213
Wu BL, Sun RP, Yang DJ (1981) The Nereidae (Polychaetous Annelids) of the Chinese Coast, Beijing
Zhao X, Li N, Guo W (2004) Further evidence for paternal inheritance of mitochondrial DNA in the sheep (Ovis aries). Heredity 93:399–403
Acknowledgments
This study was supported by the Ecological Monitoring Program for the Mai Po and Inner Deep Bay Ramsar Site under the contact No. AFCD/SQ/28/01-07, the Knowledge Innovation Program of the Chinese Academy of Sciences under contract SQ201202 and National Natural Science Foundation of China under contract 41006092 and 41130855. We would like to thank our team members in the Laboratory of Environmental Toxicology, the University of Hong Kong and the field working group for logistic support on sampling transport and assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, PP., Gu, JD. Genetic population structure of polychaeta Neanthes glandicincta (Nereididae) of the Mai Po Inner Deep Bay Ramsar Site, Hong Kong. Ecotoxicology 24, 1557–1565 (2015). https://doi.org/10.1007/s10646-015-1465-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1465-1