Abstract
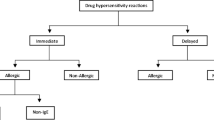
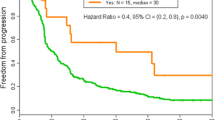
Infusion reactions (IRs) to anti-neoplastic agents require prompt recognition and immediate treatment to avert significant complications. We conducted a retrospective review of the medical records of consecutive patients who received anti-neoplastic therapy in the outpatient treatment center of the Department of Investigational Cancer Therapeutics from January 1, 2013 to November 30, 2013. Of the 597 patients who received treatment, 9 (1.5 %) had IRs (all ≤ grade 2). The most common IRs observed on first occurrence were chills (n = 5), itching, rash, and facial flushing (n = 3 each). There were no IR-related deaths. All the IRs were reversible with appropriate symptomatic treatment and the therapy was completed after temporary cessation of infusion in 7 of the 9 patients. The infusion was stopped in 2 patients due to symptoms suggestive of IgE-mediated allergic reaction and cytokine storm. Five of the 8 patients who were re-challenged with the same therapy developed a similar reaction. However, the infusion was completed in 4 of the 5 patients after administration of intravenous diphenhydramine and/or hydrocortisone, or slowing the rate of infusion. And, subsequent cycles with the same agents were uneventful. IRs to anti-neoplastic agents are rare. Though the clinical presentations are overlapping, most IRs are not IgE-mediated allergic reactions. Appropriate premedication and slow rate of infusion facilitates uneventful administration of the anti-neoplastic agents in subsequent cycles. Further study in a larger cohort of patients to identify biomarkers of hypersensitivity is warranted.
Similar content being viewed by others
References
Castells MC, Matulonis U (2015) Infusion reactions to systemic chemotherapy. UpToDate
Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J (1999) Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 17(4):1141–1145
Shlebak AA, Clark PI, Green JA (1995) Hypersensitivity and cross-reactivity to cisplatin and analogs. Cancer Chemother Pharmacol 35(4):349–351. doi:10.1007/Bf00689458
Polyzos A, Tsavaris N, Kosmas C, Arnaouti T, Kalahanis N, Tsigris C, Giannopoulos A, Karatzas G, Giannikos L, Sfikakis PP (2001) Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology 61(2):129–133. doi:10.1159/000055363
Gowda A, Goel R, Berdzik J, Leichman CG, Javle M (2004) Hypersensitivity reactions to oxaliplatin: incidence and management. Oncology (Williston Park) 18(13):1671–1675 discussion 1676, 1680, 1683–1674
Shepherd GM (2003) Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol 24(3):253–262. doi:10.1385/CRIAI:24:3:253
Baldo BA (2014) Side effects of cytokines approved for therapy. Drug Saf 37(11):921–943. doi:10.1007/s40264-014-0226-z
Joerger M (2012) Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol 23(Suppl 10):x313–x319. doi:10.1093/annonc/mds314
Chung CH (2008) Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 13(6):725–732. doi:10.1634/theoncologist.2008-0012
Zanotti KM, Markman M (2001) Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf 24(10):767–779
Weiss RB (1992) Hypersensitivity reactions. Semin Oncol 19(5):458–477
Polyzos A, Tsavaris N, Gogas H, Souglakos J, Vambakas L, Vardakas N, Polyzos K, Tsigris C, Mantas D, Papachristodoulou A, Nikiteas N, Karavokyros JG, Felekouras E, Griniatsos J, Giannopoulos A, Kouraklis G (2009) Clinical features of hypersensitivity reactions to oxaliplatin: a 10-year experience. Oncology 76(1):36–41. doi:10.1159/000178163
Maindrault-Goebel F, Andre T, Tournigand C, Louvet C, Perez-Staub N, Zeghib N, De Gramont A (2005) Allergic-type reactions to oxaliplatin: retrospective analysis of 42 patients. Eur J Cancer 41(15):2262–2267. doi:10.1016/j.ejca.2005.06.021
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME, Kerr I, Vermorken JB, Buser K, Colombo N et al (1994) European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 12(12):2654–2666
Lenz HJ (2007) Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12(5):601–609. doi:10.1634/theoncologist.12-5-601
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400. doi:10.1038/nrd1381
Mehra R, Cohen RB, Burtness BA (2008) The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol 6(10):742–750
Kang SP, Saif MW (2007) Infusion-related and hypersensitivity reactions of monoclonal antibodies used to treat colorectal cancer--identification, prevention, and management. J Support Oncol 5(9):451–457
Vogel WH (2010) Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 14(2):E10–E21. doi:10.1188/10.CJON.E10-E21
Baldo BA (2013) Adverse events to monoclonal antibodies used for cancer therapy focus on hypersensitivity responses. Oncoimmunology 2(10):e26333. doi:10.4161/onci.26333
NCI (2009) Common terminology criteria for adverse events (CTCAE) Version 4.0. NIH
Syrigou E, Syrigos K, Saif MW (2008) Hypersensitivity reactions to oxaliplatin and other antineoplastic agents. Curr Allergy Asthma Rep 8(1):56–62
Kim ES (2015) The future of molecular medicine: biomarkers, batTLEs, and big data. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting 35:22–27. doi:10.14694/EdBook_AM.2015.35.22
Wheler JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, Fu S, Moulder S, Stephen B, Wen S, Kurzrock R (2012) Risk of serious toxicity in 1181 patients treated in phase I clinical trials of predominantly targeted anticancer drugs: the M. D. Anderson cancer center experience. Ann Oncol 23(8):1963–1967. doi:10.1093/annonc/mds027
Campbell P, Marcus R (2003) Monoclonal antibody therapy for lymphoma. Blood Rev 17(3):143–152
Osterborg A, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmen A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJS, Padmanabhan S, Kozak T, Chan G, Davis RL, Losic N, Russell CA, Piotrowska M, Wilms J, Wierda WG (2008) Ofatumumab (HuMax-CD20), a novel CD20 monoclonal antibody, is an active treatment for patients with CLL refractory to both fludarabine and alemtuzumab or bulky fludarabine-refractory disease: results from the planned interim analysis of an international pivotal trial (abstr. Blood 112(11):126–127
Markman M (2003) Managing taxane toxicities. Support Care Cancer 11(3):144–147. doi:10.1007/s00520-002-0405-9
Lee C, Gianos M, Klaustermeyer WB (2009) Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol 102(3):179–187 . doi:10.1016/S1081-1206(10)60078-6quiz 187–179, 222
Timoney J, Chung KY, Park V, Trocola R, Peake C, Saltz LB , (2006) Cetuximab use without chronic antihistamine premedication. In: 2006 ASCO Annual Meeting Proceedings (Post-Meeting Edition), Vol 24, No 18S (June 20 Supplement): 13521
Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, Aguilar EA, Aapro MS, Esser R, Loos AH, Siena S (2008) Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL study. J Clin Oncol 26(33):5335–5343. doi:10.1200/JCO.2008.16.3758
Byrd JC, Waselenko JK, Maneatis TJ, Murphy T, Ward FT, Monahan BP, Sipe MA, Donegan S, White CA (1999) Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: association with increased infusion-related side effects and rapid blood tumor clearance. J Clin Oncol 17(3):791–795
Baldo BA, Pham NH (2013) Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev 32(3–4):723–761. doi:10.1007/s10555-013-9447-3
Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA (2008) Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358(11):1109–1117. doi:10.1056/NEJMoa074943
Weiss RB, Bruno S (1981) Hypersensitivity reactions to cancer chemotherapeutic agents. Ann Intern Med 94(1):66–72
Berroa F, Lafuente A, Javaloyes G, Ferrer M, Moncada R, Goikoetxea MJ, Urbain CM, Sanz ML, Gastaminza G (2014) The usefulness of plasma histamine and different tryptase cut-off points in the diagnosis of peranaesthetic hypersensitivity reactions. Clin Exp Allergy 44(2):270–277. doi:10.1111/cea.12237
Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Vanderzwan JK, Vanderlinden PWG (1994) Development of a new, more sensitive immunoassay for human tryptase - use in systemic-anaphylaxis. J Clin Immunol 14(3):190–204. doi:10.1007/Bf01533368
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Potential conflict of interest
JH serves as a consultant for Baxter; DH received honoraria for a talk and research funding from Lilly; DK received research funding from Phosplatin Pharma Inc.; FMB received honoraria from Genentech, Roche Diagnostics, and Sysmex, served as a consultant for Genentech, Novartis, Roche, and Inflection Biosciences, and received research funding from Novartis, Astra Zeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, and Bayer; MB, SB, SF, BS, KH, and AN have no conflict of interest to disclose.
Funding source
This work was supported by the NIH Clinical and Translational Science Award UL1 RR024148 and by the NIH Cancer Center Support Grant (CCSG) award CA016672 to MD Anderson Cancer Center
Ethical approval
The study was conducted with the approval of and in accordance with the guidelines of the MD Anderson Cancer Center Institutional Review Board.
Additional information
Manojkumar Bupathi and Joud Hajjar contributed equally for this manuscript.
Rights and permissions
About this article
Cite this article
Bupathi, M., Hajjar, J., Bean, S. et al. Incidence of infusion reactions to anti-neoplastic agents in early phase clinical trials: The MD Anderson Cancer Center experience. Invest New Drugs 35, 59–67 (2017). https://doi.org/10.1007/s10637-016-0395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0395-y