Abstract
Purpose
The aims of this retrospective study were to compare the results of recommended screening tests for hydroxychloroquine-related retinal toxicity and analyze disparities between the structural and functional findings.
Methods
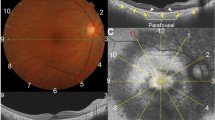
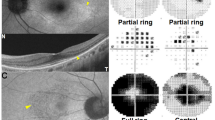
Thirty-four patients (31 women and 3 men) were included in the study. All were evaluated with standard automated perimetry using the 10-2 and/or 24-2 visual field program (Zeiss, Meditec), multifocal electroretinography (mfERG), spectral-domain optical coherence tomography (SD-OCT), and short-wavelength fundus autofluorescent imaging (SW-FAF). The results for the right eye from each patient were analyzed. Visual fields were classified as normal or abnormal based on pattern deviation plots, and mfERGs based on a comparison of R5 ring ratios to values from 20 controls. The SW-FAF images were examined for areas/rings of abnormal hypo- and/or hyperautofluorescence, and the SD-OCT line scans were classified as abnormal based on visual inspection and thickness measurements of the outer segment plus retinal pigment epithelial layer and total receptor layers compared to mean thicknesses from 35 controls.
Results
Fifteen patients had abnormal results on at least one test; however, only two patients had abnormal results on all four tests. Excluding SW-FAF, seven of the 15 had abnormal visual fields, mfERG ring ratios, and SD-OCTs. The remaining eight had either abnormal mfERGs and/or visual fields and normal SD-OCTs. We found no evidence of abnormal SD-OCTs in the presence of normal mfERG and visual field results.
Conclusions
The findings suggest that functional deficits precede structural changes seen on SD-OCT in these patients.







Similar content being viewed by others
References
Easterbrook M (1992) Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol 27(5):237–239
Michaelides M, Stover NB, Francis PJ, Weleber RG (2011) Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol 129(1):30–39
Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA (2013) Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol 131(9):1187–1197
Easterbrook E (1988) Ocular effects and safety of antimalarial agents. Am J Med 85:23–29
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF (2011) American academy of ophthalmology revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118(2):415–422
Marmor MF, Melles RB (2014) Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 121(6):1257–1262
Dale EA, Hood DC, Greenstein VC, Odel JG (2010) A comparison of multifocal ERG and frequency domain OCT changes in patients with abnormalities of the retina. Doc Ophthalmol 120(2):175–186
Talamini CL, Raza AS, Dale EA, Greenstein VC, Odel JG, Hood DC (2011) Abnormal multifocal ERG findings in patients with normal-appearing retinal anatomy. Doc Ophthalmol 123(3):187–192
Hood DC, Frishman LJ, Saszik S, Viswanathan S (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43(5):1673–1685
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM (2012) ISCEV standard for clinical multifocal electroretinography (mfERG). Doc Ophthalmol 124(1):1–13
Lyons JS, Severns ML (2007) Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol 143(5):801–809
Adam MK, Covert DJ, Stepien KE, Han DP (2012) Quantitative assessment of the 103-hexagon multifocal electroretinogram in detection of hydroxychloroquine retinal toxicity. Br J Ophthalmol 96(5):723–729
Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG (2009) Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 50(5):2328–2336
Hood DC, Cho J, Raza AS, Dale EA, Wang M (2011) Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci 88(1):113–123
Delori F, Greenberg JP, Woods RL et al (2011) Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci 52(13):9379–9390
Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, Major JC (2010) Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol 21(4):1151–1158
Anderson C, Blaha GR, Marx JL (2011) Humphrey visual field findings in hydroxychloroquine toxicity. Eye 25(12):1535–1545
Marmor MF, Chien FY, Johnson MW (2013) Value of red targets and pattern deviation plots in visual field screening for hydroxychloroquine retinopathy. JAMA Ophthalmol 131(4):476–480
Kellner U, Renner AB, Tillack H (2006) Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci 47(8):3531–3538
Maturi RK, Yu M, Weleber RG (2004) Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol 122(7):973–981
Lai TY, Chan WM, Li H, Lai RY, Lam DS (2005) Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol 140(5):794–807
Acknowledgments
The study was supported in part by grants from the National Eye Institute NIH R01 EY09076, EY018213, Foundation Fighting Blindness and a grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
Conflict of interest
The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenstein, V.C., Amaro-Quireza, L., Abraham, E.S. et al. A comparison of structural and functional changes in patients screened for hydroxychloroquine retinopathy. Doc Ophthalmol 130, 13–23 (2015). https://doi.org/10.1007/s10633-014-9474-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-014-9474-6