The reaction of 1-(cyclohexen-1-yl)pyrrolidines with 3-(α-chlorobenzyl)quinoxalin-2(1H)-ones resulted in the formation of 8,9,10,11-tetrahydroindolo[1,2-a]quinoxalin-6(5H)-ones via a tandem sequence of Stork enamine alkylation and intramolecular annulation. Oxidative dehydrogenation gave indolo[1,2-a]quinoxalin-6(5H)-one.



Similar content being viewed by others
Notes
Signal of the minor isomer.
References
(a) Mamedov, V. A. Quinoxalines. Synthesis, Reactions, Mechanisms and Structure; Springer International Publishing, 2016. (b) The Chemistry of Heterocyclic Сompounds: Condensed Pyrazines; Cheeseman, G. W. H.; Cookson, R. F., Eds.; John Wiley & Sons: New York, 1979, Vol. 35.
(a) Alleca, S.; Corona, P.; Lorigo, M.; Paglietti, G.; Loddo, R.; Mascia, V.; Busonera, B.; La Colla, P. Farmaco 2003, 58, 639. (b) Patel, M.; Mc Hugh, R. J.; Cordova, B. C.; Klabe, R. M.; Erickson-Vitanen, S.; Trainor, G. L.; Rodgers, J. D. Bioorg. Med. Chem. Lett. 2000, 10, 1729. (c) Guillon, J.; Dallemagne, P.; Pfeiffer, B.; Renard, P.; Manechez, D.; Kervran, A.; Rault, S. Eur. J. Med. Chem. 1998, 33, 293. (d) Kim, K. S.; Qian, L.; Bird, J. E.; Dickinson, K. E. J.; Moreland, S.; Schaeffer, T. R.; Waldron, T. L.; Delaney, C. L.; Weller, H. N.; Miller, A. V. J. Med. Chem. 1993, 36, 2335.
(a) Jacobsen, E. J.; Stelzer, L. S.; Belonga, K. L.; Carter, D. B.; Im, W. B.; Sethy, V. H.; Tang, A. H.; VonVoigtlander, P. F.; Petke, J. D. J. Med. Chem. 1996, 39, 3820. (b) Davey, D. D.; Erhardt, P. W.; Cantor, E. H.; Greenberg, S. S.; Ingebretsen, W. R.; Wiggins, J. J. Med. Chem. 1991, 34, 2671. (c) Colotta, V.; Cecchi, L.; Catarzi, D.; Filacchioini, G.; Martini, C.; Tacchi, P.; Lucacchini, A. Eur. J. Med. Chem. 1995, 30, 133.
(a) Sakata, G.; Makino, K.; Kurasawa, Y. Heterocycles 1988, 27, 2481. (b) Seitz, L. E.; Suling, W. J.; Reynolds, R. C. J. Med. Chem. 2002, 45, 5604. (c) Gazit, A.; App, H.; McMahon, G.; Chen, J.; Levitzki, A.; Bohmer, F. D. J. Med. Chem. 1996, 39, 2170. (d) Ali, M. M.; Ismail, M. M. F.; El-Gaby, M. S. A.; Zahran, M. A.; Ammar, Y. A. Molecules 2000, 5, 864. (e) Campiani, G.; Nacci, V.; Corelli, F.; Anzini, M. Synth. Commun. 1991, 21, 1567. (f) Kher, S. S.; Penzo, M.; Fulle, S.; Ebejer, J. P.; Finn, P. W.; Blackman, M. J.; Jirgensons, A. Chem. Heterocycl. Compd. 2015, 50, 1457. [Khim. Geterotsikl. Soedin. 2014, 1583.]
(a) Mamedov, V. A.; Kalinin, A. A. Chem. Heterocycl. Compd. 2010, 46, 641. [Khim. Geterotsikl. Soedin. 2010, 803.] (b) Kalinin, A. A.; Mamedov, V. A. Chem. Heterocycl. Compd. 2011, 46, 1423. [Khim. Geterotsikl. Soedin. 2010, 1763.]
Mamedov, V. A.; Zhukova, N. A. In Progress in Heterocyclic Chemistry; Gribble, G. W.; Joule, J. A., Ed.; Elsevier: Oxford, 2012, Vol. 24, p. 55.
Mamedov, V. A.; Zhukova, N. A. In Progress in Heterocyclic Chemistry; Gribble, G. W.; Joule, J. A., Ed.; Elsevier: Oxford, 2013, Vol. 25, p. 1.
Corona, P.; Vitale, G.; Loriga, M.; Paglietti, G.; La Colla, P.; Collu, G.; Sanna, G.; Loddo, R. Eur. J. Med. Chem. 2006, 41, 1102.
(а) Mamedov, V. A.; Kalinin, A. A. Russ. Chem. Rev. 2014, 83, 820. [Usp. Khim. 2014, 83, 820.] (b) Mamedov, V. A.; Kalinin, A. A., Balandina A. A., Rizvanov, I. Kh.; Latypov, Sh. K. Tetrahedron 2009, 65, 9412. (c) Kalinin, A. A.; Voloshina, A. D.; Kulik, N. V.; Zobov, V. V.; Mamedov, V. A. Eur. J. Med. Chem. 2013, 66, 345.
(а) Somei, M.; Yamada, F. Nat. Prod. Rep. 2003, 20, 216. (b) Gupta, L.; Talwar, A.; Chauhan, P. M. S. Curr. Med. Chem. 2007, 14, 1789.
(а) Xu, H.; Lv, M. Curr. Pharm. Des. 2009, 15, 2120. (b) Ran, J. Q.; Huang, N.; Xu, H.; Yang, L. M.; Lv, M.; Zheng, Y. T. Bioorg. Med. Chem. Lett. 2010, 20, 3534. (c) Williams, J. D.; Chen, J. J.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 2004, 47, 5753.
(а) Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Landi, L.; Prata, C.; Berridge, M. V.; Grasso, C.; Fiebig, H. H.; Kelter, G.; Burger, A. M.; Kunkel, M. W. J. Med. Chem. 2008, 51, 4563. (b) Mahboobi, S.; Sellmer, A.; Eichhorn, E.; Beckers, T.; Fiebig, H. H.; Kelter, G. Eur. J. Med. Chem. 2005, 40, 85. (с) Girgis, A. S. Eur. J. Med. Chem. 2009, 44, 1257.
Mathada, B. S. D.; Mathada, M. B. H. Chem. Pharm. Bull. 2009, 57, 557.
(а) Bhati, S. K.; Kumar, A. Eur. J. Med. Chem. 2008, 43, 2323. (b) Fakhr, I. M. I.; Radwan, M. A. A.; El-Batran, S.; Abd El-Salam, O. M. E.; El-Shenawy, S. M. Eur. J. Med. Chem. 2009, 44, 1718.
(а) Xu, H.; Fan, L. L. Eur. J. Med. Chem. 2011, 46, 1919. (b) Verma, A. K.; Jha, R. R.; Sankar, V. K.; Aggarwal, T.; Singh, R. P.; Chandra, R. Eur. J. Org. Chem. 2011, 6998. (c) Agarwal, P. K.; Sawant, D.; Sharma, S.; Kundu, B. Eur. J. Org. Chem. 2009, 292. (d) Lin, P. T; Salunke, D. B.; Chen, L. H.; Sun, C. M. Org. Biomol. Chem. 2011, 9, 2925. (e) Sokolova, E. A; Festa, A. A. Chem. Heterocycl. Compd. 2016, 52, 219. [Khim. Geterotsikl. Soedin. 2016, 52, 219.]
(а) Maiti, B.; Sun, C. M. New J. Chem. 2011, 35, 1385. (b) Lai, J. J.; Salunke, D. B.; Sun, C. M. Org. Lett. 2010, 12, 2174.
Fan, Y. S.; Jiang, Y. J.; An, D.; Sha, D.; Antilla, J. C.; Zhang, S. Org. Lett. 2014, 16, 6112.
Wang, L.; Guo, W.; Zhang, X. X.; Xia, X. D.; Xiao, W. D. Org. Lett. 2012, 14, 740.
(а) Yi, C. S.; Yun, S. Y. J. Am. Chem. Soc. 2005, 127, 17000. (b) Patil, N. T.; Lakshmi, P. G. V. V.; Singh, V. Eur. J. Org. Chem. 2010, 4719.
(а) Zhou, Y.; Ji, X.; Liu, G.; Zhang, D.; Zhao, L.; Jiang, H.; Liua, H. Adv. Synth. Catal. 2010, 352, 1711. (b) Patil, N. T.; Kavthe, R. D.; Shinde, V. S.; Sridhar, B. J. Org. Chem. 2010, 75, 3371.
(а) Rustagi, V.; Tiwari, R.; Verma, A. K. Eur. J. Org. Chem. 2012, 4590. (b) Rustagi, V.; Aggarwal, T.; Verma, A. K. Green Chem. 2011, 13, 1640.
(а) Luo, X.; Chenard, E.; Martens, P.; Cheng, Y. X.; Tomaszewski, M. J. Org. Lett. 2010, 12, 3574. (b) Abbiati, G.; Beccalli, E. M.; Broggini, G.; Paladino, G,; Rossia, E. Synthesis 2005, 2881.
(а) Maddirala, S. J.; Basanagoudar, L. D. Synth. Commun. 2003, 33, 851. (b) Beach, M. J.; Hope, R.; Klaubert, D. H.; Russel R. K. Synth. Commun. 1995, 25, 2165. (с) Yuan, Q.; Ma, D. J. Org. Chem. 2008, 73, 5159.
Huang, A.; Liu, F.; Zhan, C.; Liu Y.; Ma, C. Org. Biomol. Chem. 2011, 9, 7351.
(a) Biswas, S.; Singh, V.; Batra, S. Tetrahedron 2010, 66, 7781. (b) Zhao, F.; Zhang, L.; Liu, H.; Zhou, S.; Liu, H. Beilstein J. Org. Chem. 2013, 9, 2463.
Chicharro, R.; Castro, S.; Reino, J. L.; Arán, V. J. Eur. J. Org. Chem. 2003, 2314.
Samala, S.; Arigela, R. K.; Kant, R.; Kundu, B. J. Org. Chem. 2014, 79, 2491.
Shvedov, V. I.; Altukhova, L. B.; Alekseev, V. V.; Grinev, A. N. Chem. Heterocycl. Compd. 1970, 6, 1255. [Khim. Geterotsikl. Soedin. 1970, 1348.]
Atfah, A.; Abu-Shuheil, M. Y.; Hill, J. Tetrahedron 1990, 46, 6483.
(a) Mamedov, V. A.; Kalinin, A. A.; Gubaidullin, A. T.; Nurkhametova, I. Z.; Litvinov, I. A.; Levin, Ya. A. Chem. Heterocycl. Compd. 1999, 35, 1459. [Khim. Geterotsikl. Soedin. 1999, 1664.] (b) Mamedov, V. A.; Zhukova, N. A.; Balandina, A. А.; Kharlamov, S. V.; Beschastnova, T. N.; Rizvanov, I. Kh.; Latypov, Sh. K. Tetrahedron 2012, 68, 7363.
(a) Mamedov, V. A.; Nuretdinov, I. A.; Sibgatullina, F. G. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1989, 38, 1292. [Izv. Akad. Nauk SSSR, Ser. Khim. 1988, 1412.] (b) Saifina, D. F.; Ganieva, V. R.; Mamedov, V. A. Russ. J. Org. Chem. 2009, 45, 1244. [Zh. Org. Khim. 2009, 45, 1252.] (c) Kalinin, A. A.; Mamedov, V. A. Chem. Heterocycl. Compd. 2004, 40, 129. [Khim. Geterotsikl. Soedin. 2004, 133.] (d) Mamedov, V. A.; Kalinin, A. A.; Azancheev, N. M.; Levin, Ya. A. Russ. J. Org. Chem. 2003, 39, 125. [Zh. Org. Khim. 2003, 39, 135.]
(a) Bagal, S. K.; Adlington, R. M.; Baldwin, J. E.; Marquez, R. J. Org. Chem. 2004, 69, 9100. (b) Maiti, S.; Achari, B.; Mukhopadhyay, R.; Banerjee, A. K. J. Chem. Soc., Perkin Trans. 1 2002, 1769. (c) McNally, J. J.; Youngman, M. A.; Lovenberg, T. W.; Nepomuceno, D. H.; Wilson, S. J.; Dax, S. L. Bioorg. Med. Chem. Lett. 2000, 10, 213.
(a) Overman, L. E.; Wolfe, J. P. J. Org. Chem. 2002, 67, 6421. (b) Mitschke, U.; Osteritz, E. M.; Debaerdemaeker, T.; Sokolowski, M.; Bauerle, P. Chem.–Eur. J. 1998, 4, 2211. (c) Ma, Li-J., Inokuchi, T. Chem. Commun. 2010, 46, 7037.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Farrugia, L. J. J. Appl. Crystallogr. 1999, 32(4), 837.
APEX (Version 2.1). SAINTPlus. Data Reduction and Correction Program. Version 7.31A. Bruker Advansed X-Ray Solutions; BrukerXS Inc.: Madison, 2006.
Spek, A. L. J. Appl. Crystallogr. 2003, 36, 7.
This work received financial support from the Russian Science Foundation (project No. 14-23-00073-p).
Author information
Authors and Affiliations
Corresponding author
Additional information
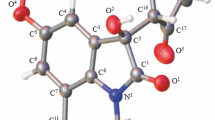
Supplementary information file containing X-ray crystallography data for compounds 3a,d–f,h,i is available from the journal website at http://link.springer.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, Submitted December 20, 2016 2017, 53(5), 560–567
Electronic supplementary material
ESM 1
(PDF 404 kb)
Rights and permissions
About this article
Cite this article
Mamedov, V.A., Khafizova, E.A., Zamaletdinova, A.I. et al. A New Method for the Synthesis of Substituted 8,9,10,11-Tetrahydroindolo[1,2-a]Quinoxalin-6(5H)-Ones. Chem Heterocycl Comp 53, 560–567 (2017). https://doi.org/10.1007/s10593-017-2090-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2090-0