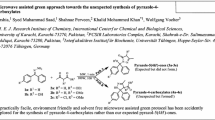
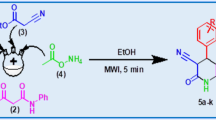
A simple and green protocol has been developed for the synthesis of benzimidazoles. In this protocol, 2-[(alkyl/aryl)(ethoxy)methylidene]hydrazinecarboxylates with 1,2-phenylenediamine derivatives under solvent- and catalyst-free conditions under microwave irradiation lead to products with good yields in short reaction times.
Similar content being viewed by others
References
K. M. Hosamani, H. R. Seetharamareddy, R. S. Keri, M. S. Hanamanthagouda, and M. G. Moloney, J. Enzyme Inhib. Med. Chem., 24, 1095 (2009).
J. Velík, V. Baliharová, J. Fink-Gremmels, S. Bull, J. Lamka, and L. Skálová, Res. Vet. Sci., 76, 95 (2004).
R. G. Kulkarnia, S. A. Laufer, V. M. Chandrashekhar, and A. Garlapati, Med. Chem., 9, 91 (2013).
K. C. S. Achar, K. M. Hosamani, and H. R. Seetharamareddy, Eur. J. Med. Chem., 45, 2048 (2010).
L. K. Soni, T. Narsinghani, and A. Sethi, Med. Chem. Res., 21, 4330 (2012).
N. R. T. Gowda, C. V. Kavitha, K. K. Chiruvella, O. Joy, K. S. Rangappa, and S. C. Raghavan, Bioorg. Med. Chem. Lett., 19, 4594 (2009).
M. Rashid, A. Husain, and R. Mishra, Eur. J. Med. Chem., 54, 855 (2012).
M. Tunçbilek, T. Kiper, and N. Altanlar, Eur. J. Med. Chem., 44, 1024 (2009).
P. Wang, G. Zheng, Y. Wang, X. Wang, H. Wei, and W. Xiang, Tetrahedron, 68, 2509 (2012).
S. Sarno, E. Papinutto, C. Franchin, J. Bain, M. Elliott, F. Meggio, Z. Kazimierczuk, A. Orzeszko, G. Zanotti, R. Battistutta, and L. A. Pinna, Curr. Top. Med. Chem., 11, 1340 (2011).
K. Kumar, D. Awasthi, S.-Y. Lee, I. Zanardi, B. Ruzsicska, S. Knudson, P. J. Tonge, R. A. Slayden, and I. Ojima, J. Med. Chem., 54, 374 (2011).
P. N. Preston, Chem. Rev., 74, 279 (1974).
A. E. Sparke, C. M. Fisher, R. E. Mewis, and S. J. Archibald, Tetrahedron Lett., 51, 4723 (2010).
J. She, Z. Jiang, and Y. Wang, Synlett, 12, 2023 (2009).
H. Sharghi, O. Asemani, and R. Khalifeh, Synth. Commun., 38, 1128 (2008).
Z.-H. Zhang, L. Yin, and Y.-M. Wang, Catal. Commun., 8, 1126 (2007).
G. Ayhan-Kılcıgil, and N. Altanlar, Farmaco, 58, 1345 (2003).
R. J. Perry and B. D. Wilson, J. Org. Chem., 58, 7016 (1993).
M. Raban, H. Chang, L. Craine, and E. Hortelano, J. Org. Chem., 50, 2205 (1985).
B. Kahveci, E. Menteşe, M. Özil, S. Ülker, and M. Ertürk, Monatsh. Chem., 144, 993 (2013).
B. Kahveci, M. Özil, and M. Serdar, Heteroatom Chem., 19, 38 (2008).
A. Shaukat, H. M. Mirza, A. H. Ansari, M. Yasinzai, S. Z. Zaidi, S. Dilshad, and F. L. Ansari, Med. Chem. Res., 22, 3606 (2012).
B. Karami, S. Khodabakhshi, and Z. Haghighijou, Chem. Papers, 66, 684 (2012).
R. C. Elderfield and K. L. Burgess, J. Am. Chem. Soc., 82, 1975 (1960).
H. Antaki and V. Petrow, J. Chem. Soc., 2873 (1951).
Y. Wang, K. Sarris, D. R. Sauer, and S. W. Djuric, Tetrahedron Lett., 47, 4823 (2006).
E. Menteşe, J. Chem. Res., 37, 168 (2013).
E. Menteşe, F. İslamoğlu, E. Bal, and B. Kahveci, Eur. J. Chem., 4, 25 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1221-1225, August, 2013.
Rights and permissions
About this article
Cite this article
Menteşe, E., Doğan, I.S. & Kahveci, B. Green Protocol: Solvent- and Catalyst-Free Synthesis of Benzimidazole Derivatives via Microwave Technique. Chem Heterocycl Comp 49, 1136–1140 (2013). https://doi.org/10.1007/s10593-013-1354-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1354-6