Abstract
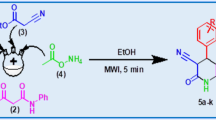
A practically efficient, environment friendly, and solvent free microwave-assisted protocol was developed for the synthesis of pyrazole-4-carboxylates. In this connection, we synthesized five new compounds (4a-4e) via microwave irradiation of a mixture of benzophenone hydrazones and acetoacetates in the absence of solvent. Short reaction time, easy workup and quantitative yield are the striking features of our protocol.
Graphical abstract





Similar content being viewed by others
References
H.A. Abdel-Aziz, A.A.I. Mekawey, K.M. Dawood, Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 44, 3637 (2009)
H.A. Abdel-Aziz, H.S.A. El-Zahabi, K.M. Dawood, Microwave-assisted synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur. J. Med. Chem. 45, 2427 (2010)
H. Kumar, D. Saini, S. Jain, N. Jain, Pyrazole scaffold: a remarkable tool in the development of anticancer agents. Eur. J. Med. Chem. 70, 248 (2013)
Y. Liu, J. Luo, P. Duan, J. Shao, B. Zhao, J. Miao, Synthesis of pyrazole peptidomimetics and their inhibition against A549 lung cancer cells. J. Bioorg. Med. Chem. Lett. 22, 6882 (2012)
I. Vujasinović, A. Paravić-Radičević, K. Mlinarić-Majerski, K. Brajša, B. Bertoša, Synthesis and biological validation of novel pyrazole derivatives with anticancer activity guided by 3D-QSAR analysis. Bioorg. Med. Chem. Lett. 20, 2101 (2012)
A.M. Farag, K.A.K. Ali, T.M.A. El-Debss, A.S. Mayhoub, A.E. Amr, N.A. Abdel-Hafez, M.M. Abdulla, Design, synthesis and structure–activity relationship study of novel pyrazole-based heterocycles as potential antitumor agents. Eur. J. Med. Chem. 45, 5887 (2010)
R. Sridhar, P.T. Perumal, S. Etti, G. Shanmugam, M.N. Ponnuswamy, V.R. Prabavathy, N. Mathivanan, Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg. Med. Chem. Lett. 14, 6035 (2004)
E. Hernández-Vázquez, R. Aguayo-Ortiz, J.J. Ramírez-Espinosa, S. Estrada-Soto, F. Hernández-Luis, Synthesis, hypoglycemic activity and molecular modeling studies of pyrazole-3-carbohydrazides designed by a CoMFA model. Eur. J. Med. Chem. 69, 10 (2013)
D.V. Dekhane, S.S. Pawar, S. Gupta, M.S. Shingare, C.R. Patil, S.N. Thore, Synthesis and anti-inflammatory activity of some new 4,5-dihydro-1,5-diaryl-1H-pyrazole-3-substituted-heteroazole derivatives. Bioorg. Med. Chem. Lett. 21, 6527 (2011)
V. Sharath, H.J. Kumar, N.J. Naik, Synthesis of novel indole based scaffolds holding pyrazole ring as anti-inflammatory and antioxidant agents. Pharm. Res. 6, 785 (2013)
P. Nagababu, J.N.L. Latha, M. Rajesh, S. Satyanarayana, DNA-binding and cytotoxicity of the cobalt(III) ethylenediamine pyrazole complex [Co(en)2(pyz)2]3+. J. Iran. Chem. Soc. 6, 145 (2009)
C.S. Hawes, C.M. Fitchett, S.R. Batten, P.E. Kruger, Synthesis and structural characterisation of a Co(II) coordination polymer incorporating a novel dicarboxy-Trögers base/bis-pyrazole mixed ligand system. Inorg. Chim. Acta 389, 112 (2012)
S. Kannan, J.S. Gamare, K.V. Chetty, M.G.B. Drew, Coordination and extraction studies of an unexplored bi-functional ligand, carbamoyl methyl pyrazole (CMPz) with uranium(VI), lanthanum(III) and cerium(III) nitrates. Polyhedron 26, 3810 (2007)
A. Alsalme, K. Al-Farhan, M. Ghazzali, M. Khair, R.A. Khan, J. Reedijk, Structure of bis(nitrato)tetrakis(pyrazole) cobalt(II): fine tuning in the stabilization of coordination entities by using intramolecular hydrogen bonding. J. Inorg. Chim. Acta 407, 7 (2013)
B. Machura, R. Kruszynski, M. Jaworska, Synthesis, crystal, molecular and electronic structure of rhenium nitrosyl with pyrazole in the coordination sphere. Inorg. Chem. Commun. 8, 960 (2005)
L. Soria, P. Ovejero, M. Cano, J.A. Campo, M.R. Torres, C. Núñez, C. Lodeiro, Selecting pyrazole-based silver complexes for efficient liquid crystal and luminescent materials. Dyes. Pigments 110, 7159 (2014)
C. Feng, X.-J. Li, D. Zhang, Z.-R. Qu, H. Zhao, Two new palladium and platinum complexes with a bidentate pyrazole-based ligand: crystal structure, fluorescence and Hirshfeld surface analysis. J. Iran. Chem. Soc. 13, 823 (2016)
J. Li, J. Zhou, Y. Li, L. Weng, X. Chen, Z. Yu, Z. Xue, Synthesis and structures of two luminescent Zn(II) complexes with pyrazole and carboxylate ligands. Inorg. Chem. Commun. 7, 538 (2004)
P. Ovejero, M.J. Mayoral, M. Cano, M.C. Lagunas, Luminescence of neutral and ionic gold(I) complexes containing pyrazole or pyrazolate-type ligands. J. Organomet. Chem. 692, 1690 (2007)
J. Chen, P. Song, J. Liao, H. Wen, R. Hong, Z. Chen, Y. Chi, Luminescent homodinuclear copper(I) halide complexes based on the 3,5-bis{6-(2,2′-dipyridyl)} pyrazole ligand. Inorg. Chem. Commun. 13, 1057 (2010)
L. Xu, C. Gu, R. Li, Y. Yu, T. Wang, A green four-component synthesis of 2-amino-3-cyano-4-aryl-6-sulfanepyrimidine in water solvent using phase-transfer catalyst. J. Iran. Chem. Soc. 13, 597 (2016)
C. Li, B.M. Trost, Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 105, 13197 (2008)
R.A. Sheldon, Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 41, 1437 (2012)
A. Zare, A. Hasaninejad, A. Khalafi-Nezhad, A. Parhami, A.R. Moosavi Zare, A solventless protocol for the Michael addition of aromatic amides to α,β-unsaturated esters promoted by microwave irradiation. J. Iran. Chem. Soc. 5, 100 (2008)
R.J. Giguere, T.L. Bray, S.M. Duncan, G. Majetich, Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 27, 4945 (1986)
F. Rodriguez, J. Francisco, Constructing molecular complexity from alkynol derivatives: a journey from Fischer carbene complexes to tandem catalysis with gold and other carbophilic Lewis acids. Synlet 24, 1757 (2013)
S. Sardar, T. Akhtar, S. Hameed, K.M. Khan, Synthesis, biological evaluation of some arylsulfonyl, carboxamide derivatives of 3-methyl-1H-pyrazol-5(4H)-one. J. Chem. Soc. Pak. 34, 1531 (2012)
K.M. Khan, G.M. Maharvi, M.T.H. Khan, S. Perveen, M.I. Choudhary, Atta-ur-Rahman, A facile and improved synthesis of sildenafil (Viagra®) analogs through solid support microwave irradiation possessing tyrosinase inhibitory potential, their conformational analysis and molecular dynamics simulation studies. Mol. Divers. 9, 15 (2005)
K.M. Khan, G.M. Maharvi, S. Perveen, M.T.H. Khan, R.J. Abdel-Jalil, S.T.A. Shah, M. Fecker, M.I. Choudhary, Atta-ur-Rahman, W. Voelter, Synthesis of methyl ether analogues of sildenafil (Viagra®) possessing tyrosinase inhibitory potential. Chem. Biodivers. 2, 470 (2005)
Acknowledgments
The authors are thankful for their financial support to Higher Education Commission (HEC), Pakistan to Project No. 20-1910 under National Research Program for Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arshia, Saad, S.M., Perveen, S. et al. Microwave-assisted green approach toward the unexpected synthesis of pyrazole-4-carboxylates. J IRAN CHEM SOC 13, 1405–1410 (2016). https://doi.org/10.1007/s13738-016-0855-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0855-5