The design, development, and biological evaluation of the multitarget series of tetrahydroindolo-[2,3-c]quinolinones is based on the “old” drug ambocarb via the “deconvolution” approach and is reviewed for the potential treatment of neurodegenerative conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The importance of heterocyclic compounds in drug discovery and development is well recognized over the last decades with multiple heterocyclic novel chemical entities approved as drugs or currently in clinical development [1, 2]. The syntheses of heterocyclic systems are comprehensively and periodically reviewed on both novel synthetic routes and novel types of the compounds, as well as their applications [3]. The goal of this review is to provide a perspective of the changing landscape on the application of both novel and known heterocyclic compounds as multifunctional pharmacophores in drug discovery and development with focus on the central nervous system (CNS) diseases and disorders. The example of multifunctional pharmacophores is discussed for the series of 2,3,4,7-tetrahydroindolo[2,3-c]quinolin-1-ones.
Underlying Concepts of Drug Design
A general assumption underlying drug discovery is that therapeutic agents with higher specificity for their molecular targets provide better efficacy and fewer side effects. Since the early 1980s, when the ability to screen compounds at receptor targets increased, drug discovery efforts traditionally focus on developing “magic bullets” – agents that provide the recognizable sharp strike against critical targets in a disease process while minimizing “by-side” damage. This “one molecule – one target” paradigm has led to the discovery of many successful drugs, and will probably remain a milestone for years to come. However, a highly selective ligand for a given target does not always result in a clinically efficacious drug for the treatment of complex diseases such as CNS disorders, which involve multiple pathogenic factors [4]. Despite the clear importance of off-target interactions with side effects, many drugs acting in the CNS (including some targeted to have high specificity) have been shown to interact with multiple targets at therapeutically relevant concentrations. But the “magic bullet” approach still continues to lead drug discovery in this area [5].
Recently, several authors have proposed that designing “selectively non-selective” drugs that interact with several molecular targets – “magic shotguns” – may lead to more effective medications for a variety of complex diseases [6–8]. For the CNS disorders, where highly complex interactions underlie normal function, drug multifunctionality is particularly relevant [9–15]. This concept is supported by both theoretical and empirical studies and is congruent with our current understanding of biology in general or how genes work. Drug promiscuity is already well-recognized among certain classes of CNS-active modulators such as general anesthetics [16, 17], anticonvulsants [18], and antipsychotics [19], and this property may extend to other therapeutic classes such as antidementia drugs [20].
Trazodone (1) is a good example of a dose-dependent multifunctional drug in psychopharmacology [21, 22]. The drug has hypnotic actions at low doses due to blockade of 5-HT2A receptors, as well as H1 histamine receptors and α1-adrenergic receptors. Higher doses involve the blockade of the serotonin transporter (SERT) and turn trazodone (1) into an antidepressant [22]. Although trazodone (1) has traditionally been used as a low-dose hypnotic, a new controlled-release formulation that has the potential to improve the tolerability of high doses may provide an opportunity to revisit this multifunctional drug as an antidepressant as well.
Another example of a multifunctional antipsychotic drug is amitifadine (2) which inhibits both binding to the serotonin, norepinephrine, and dopamine transporters (SERT, NET, and DAT, respectively) and reuptake of the respective biogenic amines [23, 24]. Results of a small clinical trial indicated that amitifadine (2) had statistically significant antidepressant effects and was well tolerated [25]. Currently, amitifadine (2) is in clinical trials for the treatment of adult attention deficit/hyperactivity disorder (ADHD).

However, sometimes it remains uncertain which subset of polypharmacological interactions is important for clinical efficacy. Potential contributors to this uncertainty include the fruitful history of linking off-target interactions with side effects, as well as the emphasis on high specificity compounds in drug development [1, 2].
Importantly, many “old” drugs have been developed by earlier (and arguably equally successful) drug discovery efforts that by necessity targeted entire organisms. Numerous drugs have been approved and marketed based on positive animal model data without a clear understanding of what the drug does at the molecular level. This “holistic” or systems-centric approach was the basis for the discovery of many highly successful drugs (e.g., valproic acid and clozapine, among others) whose putative targets are only recently coming to light. No surprise, screening these older drugs using panels of conventional biochemical assays indicate that many of them are promiscuous and exhibit activity against a wide range of molecular targets. In fact, it is now commonly accepted that the polypharmacology of these drugs (i.e., their ability to modulate the activity of multiple protein targets) is at least partly responsible for their efficacy, and they can be viewed as “magic shotguns” [6, 26].
Thus, perhaps an ideal drug may be one whose efficacy is based not on the modulation of a single target, but rather on the rebalancing of several proteins or events that contribute to the etiology, pathogeneses, and progression of the disease – a multitarget drug.
Approaches to Multitarget Drugs
Three strategies have been proposed for the multitarget drugs [27, 28]: (1) multidrug combinations or “cocktail” prescribing multiple individual medications, (2) development of a “single-pill drug combination”, and (3) design of a single compound with selective polypharmacology. The first two approaches are confronted by drug–drug interactions, patent compliance, and pharmacopeia guidelines. This discussion will be focused on the third approach, which is challenged by the validation of target combinations and development of the structure–activity relationship (SAR) for several targets.
In recent years, there has been an increasing trend in medicinal chemistry for the deliberate and rational design of drugs acting selectively at multiple targets simultaneously – “designed multiple ligands” [29, 30]. The designed multiple-ligand strategy involves combining pharmacophores from two or more selective ligands either by the addition of a linker to form a conjugate or by taking advantage of structural commonalities to overlap pharmacophores [29]. Overlapping pharmacophores holds more promise in CNS drug development because of the high degree of structural similarity among both potential molecular targets and their ligands. This structural similarity is also a major disadvantage because designing drugs capable of targeting only a subset of similar molecules is exceedingly difficult.
Figure 1 exemplifies the overlapping pharmacophores. In fact, this approach represents the fragment-based ligand design [31]. The starting point is the selection of several templates (Template 1, 2, 3, etc.) each separately binding with high affinity at the specific target(s) (Targets 1, 2, 3, etc.). The next step is integrating the parent templates into the hybrid Polycyclic Template, which potentially retains some of their key parent structural and, hopefully, pharmacological effects.
The synthesis of a limited number of compounds within the Polycyclic Template followed by in vitro testing these compounds in the biochemical assays at the selected Targets 1, 2, 3, etc. would allow a rapid evaluation of whether the design hypothesis works for the Polycyclic Template. Objectives for the Polycyclic Template should be carefully defined for CNS-active drugs and may include (1) elimination of unwanted activities in a design template, (2) integration of a novel action(s) into design template, and (3) maintaining a small size, modest lipophilicity, and a limited polar surface. These objectives require broad and deep involvement of various teams of experts with a clear understanding of the subset pharmacology of the parent templates along with comprehensive expertise in medicinal and computational chemistry.
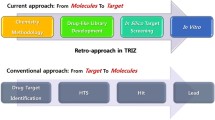
A different approach to the polyfunctional pharmacophores may involve exploration of the “old holistic” drugs that have been developed on positive animal model data without a clear understanding of what the drug does at the molecular level. One can virtually “deconvolute” the structure of a drug with the established in vivo profile and also virtually analyze the known pharmacological properties of the unit subsets of deconvolution (Fig. 2).
Retro application of the virtual analysis data to the studied drug may result in (1) elucidation of the mechanism(s) of action, (2) novel application(s) based on the mechanism(s) of action (drug repurposing), and (3) designing novel analogs with potentially improved pharmacological profiles.
Tetrahydroindolo[2,3-c]quinolinone Compounds as Multifunctional Pharma-Cophores
The strategy in Figure 2 was implemented to explore ambocarb (3, 3,3,6-trimethyl-2,3,4,7-tetrahydro-1H-indolo[2,3-c]quinolin-1-one), a nootropic drug developed through clinical trials in the early 1990s in Ukraine [32–35].
Ambocarb (sometimes referred to also as carbacetam) was designed and developed as the β-carboline derivative with the hypothesis based on numerous investigations that demonstrated some of the β-carboline derivatives to be useful tranquilizers, anxiolytics, and stress protectors [36, 37]. These actions are generally thought to be mediated by modulation of the GABAA receptors, specifically, the benzodiazepine receptors of the GABAA–chloride channel receptor complex [36, 37]. In rodents, ambocarb (3) has demonstrated a low dose efficacy in the models of survival under traumatic shock and hypoxia, and in the models of memory improvement [33, 34]. In clinical trials, ambocarb (3) was effective as a nootropic drug, improving brain recovery after various pathological events. At daily doses of 40-80 mg, ambocarb (3) improved the recovery of brain function after cranial trauma and following cerebral damage induced by intoxication [35, 38].
In vitro and in vivo studies with ambocarb (3) on the mechanism of action involved the GABAA receptors [39], sodium channels [40], and NMDA [41] and non-NMDA [42] receptors. None of the studies clarified the mechanism of action of ambocarb (3) at therapeutically relevant concentrations for the dose range in animal models and in humans [35, 38]; thus, the mechanism of action of ambocarb (3) as a neuroprotective and cognitive-enhancing drug remained unknown. In addition, only a limited number of the derivatives of ambocarb (3) have been reported in the literature [32–34, 43–46]. Thus, the nootropic drug ambocarb (3) seemed to be a good model for testing the “deconvolution” hypothesis (Fig. 2) as the approach to multitarget pharmacophores.
Virtual deconvolution of ambocarb (3) logically resulted in four template subsets A-D (Fig. 3).
Each of the subsets A-D was first analyzed by PASS (Prediction of Activity Spectra for Substances), a computer program developed by Poroikov, Filimonov, and co-workers [47–51]. The PASS approach is built on the hypothesis that the biological activity of any organic compound is a function of its structure. PASS evaluation of the biological activity spectra for novel compounds is based on the SAR Base (Structure-Activity Relationship data and knowledge base), which accumulates the results of the training set analysis. In the most recent version, PASS predicts over 4400 biological activities with a mean accuracy of 95%. The list of predicted biological activities comprises the pharmacological effects, biochemical mechanisms of action, specific toxicities, antitargets, substrates associated with the drug metabolism, substrates associated with the drug transport, and substrates associated with the gene expression. Examples of successful PASS application to discovery of compounds with the predicted pharmacological activities and/or targets and confirmed in vivo effects have been described in the literature [52–55].
Based on the data generated by PASS for the subsets A-D, literature mining was carried out for the factual data reported on the numerous derivatives in each subset. Powerful search capabilities are offered by PubMed, and the search was performed with the most effective use of Boolean combinations, filters of several kinds, and the rich set of medical subject headings (MeSH) [56]. For ambocarb, PASS prediction resulted in a combination of the targets/pharmacological activities of the subsets A-D.
Finally, for each of the subsets A-D the data were generalized by the most common factual biological activities. The results are presented in Fig. 4 (A-D) as 3D graph correlations of the receptors/targets and the most common in vivo pharmacological effects/therapeutic areas. The green and yellow shapes on the Target plane for the subsets A-D represent the targets for the potential treatment of certain conditions (CNS and non-CNS, respectively), while the red shapes indicate the targets for the potential/established undesired side effects.
The next step involved in vitro evaluation of ambocarb for the factual biological targets. Ambocarb was screened at 10 μM concentration against 227 CNS targets – ion channels, receptors, enzymes and transporters in radioligand binding and cell functional assays (Brain Panel/Organ Safety Profile at CEREP [www.cerep.fr]), and against 442 kinases in the competitive binding assays (KINOMEscan [www.kinomescan.com]), with follow-on determination of the IC50/Ki(KD) values for the discovered targets [57–63]. The factual in vitro binding and functional profile of ambocarb (3) is presented in Figure 4 (E) and clearly indicates the preservation of some CNS binding/functional targets of the structural subsets A-D (green ovals on the Target plane, MT3/QR2, DYRK1A) as well as the additional CNS targets (neuronal L-type calcium channels, GRK2, non-benzodiazepine GABAA). The dotted circle encompasses some possible therapeutics indications related to the identified targets. Importantly, none of the “undesired” targets of the subsets – those that may be responsible for the potential side effects – appeared in the in vitro profile of ambocarb (crossed red shapes).
The values of IC50/Ki(KD) ≤ 1 μM have been determined for the MT3(ML2)/QR2 receptor/enzyme (~ 5 nM) [63], neuronal L-type calcium channels (~ 750 nM) [57, 61], and DYRK1A kinase (~ 950 nM) [59, 60], and the rest of the targets were confirmed in a range of 5-7 μM (Fig. 4, E). The latter range can hardly contribute to the in vivo pharmacological profile of ambocarb based on the pharmacokinetic profile in animals [59, 60] and the efficacy drug levels in animals and humans [33–35, 38], but certainly could be reasonably explored at the higher doses.
Thus, ambocarb (3) appears to be the multitarget CNS ligand, and its mechanisms of action may involve triple-target and dose-dependent preferential interactions with the melatonin MT3(ML2)/QR2 receptor/enzyme, neuronal L-type calcium channels, and DYRK1A kinase at the therapeutically relevant drug levels [35, 38]. The target overview, in vitro SAR, and in vivo data for ambocarb (3) and its novel analogs are presented further in this discussion.
In the next step, the series of novel analogs of ambocarb (3) were designed, synthesized, and evaluated in vitro and in vivo.
Design and Synthesis of Tetrahydroindolo[2,3-c]quinolinones
The early synthesis of ambocarb (3) and its several analogs was based on the well-developed synthetic approach via the oxonium cations of type 5 [32–34, 43–46]. The major advantage of the oxonium cations is their easy oxygen-to-nitrogen exchange with convenient access to the nitrogen heterocycles [64]. However, while being an excellent laboratory procedure for the synthesis of pyridines, isoquinolines, and other condensed nitrogen heterocycles and carbocycles, this approach is unacceptable for process chemistry due to the use of the explosive oxidant, 70% aqueous perchloric acid, for the preparation of acetyl perchlorate at the first step, and the gaseous ammonia at the next step, which results in highly hazardous production conditions.

In addition, the earlier reported several analogs of ambocarb (3) were synthesized only with a limited variation of the substituents at positions 1 (oximes or reduction), 3 (unsubstituted), or 6 (alkyl, benzyl, aryl) [32–34, 43–46]. No SAR exploration has been reported for substitution at positions 8-11. For the synthesis of novel analogs substituted at positions 9-11 along with a variation of the substituents at positions 3 and 6, the synthetic approach was first developed for the starting indoles 4, 11.
The synthetic scheme is based on commercially available ortho-substituted benzoic acids 6 which are converted into dicarboxylic compounds 7, followed by convenient cyclization into indoles 8 and deacylation to indolones 9. Condensation of compounds 9 with cyclohexane-1,3-diones to substituted indoles 10, followed by additional deacylation, resulted in the desired indoles 4, 11 in a total yield of 40-60% [57, 58, 60].

Development of the process chemistry procedure for the synthesis of ambocarb (3) and novel compounds 14 from diketones 4, 11 eliminated the use of chlorinated chemicals, implemented mild conditions, and resulted in an increase of the total yield of compounds 3, 14 by 13-35% in comparison with the conventional method with the use of perchloric acid [57, 58, 60]. In this procedure, it is not necessary anymore to isolate the oxonium salts 12, and the products of their hydrolysis in situ, diketones 13, are directly converted into the desired indoloquinolinones 3, 14 (Table 1). The N(7)-alkylated compounds 15a-c were prepared by a standard alkylation procedure [57].

Exploratory Studies with Tetrahydroindolo[2,3-c]quinolinones
Over 40 drug-like analogs of ambocarb (3) have been prepared for in vitro and in vivo evaluation [57, 58]. In vitro studies with the compounds 14 (Table 1) demonstrated that minor structural modifications to the tetrahydroindolo[2,3-c]quinolinone pharmacophore sensitively rebalance the in vitro triple-target binding profile in the series, which may lead to modification of the corresponding in vivo pharmacological profiles. Overall, the in vitro binding trend in the series 14 at MT3/QR2 receptor/enzyme, neuronal L-type calcium channels, and DYRK1A kinase was maintained, while the in vitro profile rebalancing depends on the substituents and their location at positions 9, 10, and 11.
Specifically, the binding affinity at MT3/QR2 was maximized to the values of IC50 < 1 nM (for example, compound 14g), while for the halo- and trifluoromethoxy-substituted compounds the binding affinity was maintained at the level of ambocarb (3) (IC50 ~ 3-5 nM) or decreased to the IC50 values > 10-100 nM. None of the tested compounds demonstrated any agonist/antagonist activity in the cell functional assays for other melatonin receptor subtypes, MT1 and MT2, at concentrations up to 10 μM.
The binding affinity at the neuronal L-type channels is also modulated by the substitution at position 9, 10, and 11, and can be significantly enhanced for some compounds in comparison with the parent compound ambocarb (3) [57]. Increased binding at L-type calcium channels while maintaining or enhancing binding at MT3/QR2 was observed for several compounds. On the other hand, ambocarb remained the most potent inhibitor of DYRK1A kinase in the series [59, 60]. The SAR direction for enhancing the inhibition of DYRK1A by tetrahydroindolo[2,3-c]quinolinone compounds was clarified after testing the harmine-like compounds in the functional assays of tau phosphorylation and is discussed below.
Thus, the initially selected in vitro evaluation of the tetrahydroindolo[2,3-c]-quinolinone compounds 14 at three targets of the parent ambocarb (3) confirmed the multitarget potential of the series. The effects at the targets of interest, additional studies, and potential applications of the tetrahydroindolo[2,3-c]quinolinone compounds are discussed below.
Inhibition of melatonin MT3/QR2 receptor/enzyme
The melatonin MT3 (ML2) receptor was first described as a cell membrane receptor in the hamster brain [65, 66]. The binding site of MT3 among the melatonin receptors is differentiated from two other subtypes, the G protein-coupled MT1 and MT2, by the significantly lower binding of melatonin (16) (~ 10 nM vs. less than 1 nM for MT1 and MT2) and a very rapid association and dissociation “ping-pong” kinetics [67, 68]. By 1999, only a dozen articles describing MT3 site were published.
MT3-binding sites were reported to be functionally coupled to phosphoinositide hydrolysis in Syrian hamster RPMI 1846 melanoma cells [68]. Further studies extended the pharmacological characterization of MT3. By using a mild extraction procedure and affinity chromatography, a single protein was isolated that, once sequenced, turned out to be a long forgotten quinone reductase 2 (QR2). Further studies on this enzyme firmly established that MT3 and QR2 are the same protein [69–72]. Most importantly, QR2–/– mice did not have any measurable MT3-binding sites [71]. Recently, the co-crystallization of QR2 with melatonin and 2-iodo-melatonin confirmed that QR2 corresponds to MT3 [73]. QR2 is the cytosolic enzyme, that participates in the protection against oxidative stress by preventing the electron transferring reaction of quinones. So far, it has been difficult on the basis of molecular data to identify clearly the physiological function of this enzyme. Inhibition of QR2 expression induces upregulation of different enzymes with antioxidant properties; inhibitors of QR2 are known to produce antioxidant and “detoxifying” effects. Recent studies indicated that QR2 may actually transform certain quinone substrates (for example, the products of dopamine oxidation) into more highly reactive compounds/radicals capable of causing cellular damage [74]. Therefore it was hypothesized that inhibition of QR2 may lead to protection of cells against highly reactive species.
Since 2000, extensive studies on QR2 functions and identification of specific MT3 binding ligands were reported by the group at Servier & Co. (France) [68–87]. A comprehensive approach to MT3/QR2 pharmacology studies may shed light on the unique catalytic properties and biological functions of QR2. For example, QR2 polymorphism has been associated with idiopathic Parkinson’s disease, schizophrenia, alcohol withdrawal symptoms, and clozapine-induced agranulocytosis.
Meanwhile, interesting findings on the in vivo effects of the selective MT3/QR2 ligands have been reported. Very recently, inhibition of QR2 was identified as a novel therapeutic strategy toward the treatment of learning and memory deficits especially observed in the aged brain [83, 88]. The main findings of the studies included the following: (1) the broad expression of QR2 in the brain with particular enrichment in neurons of the hippocampus and cortex in rodents and humans; (2) significant increase of QR2 level in patients with Alzheimer’s disease; (3) the ability of selective QR2 inhibitors to act as neuroprotective agents in two models of neurotoxicity in vitro and to reverse the proapoptotic action of QR2; (4) the capacity of these inhibitors to facilitate learning behaviors in rodents; and (5) the facilitated cognitive abilities of QR2–/– mice in various learning tasks.
Together, these data strongly suggest that QR2 should be considered as a novel and potent modulator of cognitive processes in the mammalian brain. Selective QR2 inhibitors should possibly be considered as cognition enhancers in the treatment of brain disorders associated with learning deficits, including various forms of dementias.
The number of MT3-selective ligands published up to date is rather limited and no clear SARs have been reported. MCA-NAT (17) is the analog of melatonin with better binding at MT3, but, similarly to melatonin, is non-selective for MT1 and MT2. Prazosin (18) is a selective MT3 ligand that is used as the control in the radioligand binding assays at CEREP, but also has a high affinity at α-adrenergic receptors. Notable highly MT3-selective compounds are 19 and S32796 (20) (both compounds have no affinity for MT1 and MT2, IC50 > 10 μM), while S29434 (21) is the most potent ligand in all tested cellular assays [77, 89].
The analysis of the X-ray structures of human QR2 (hQR2) in complex with the ligand 19, the compound with the highest binding affinity, and less potent bicyclic compounds provides further information for the development of more effective molecular ligands of hQR2 [86]. Two major factors of the increased affinity of inhibitors to hQR2 were observed. First, the QR2 active site is generally hydrophobic in nature with few polar side chains, and the oxidized FAD cofactor presents a flat molecular surface conducive to π–π interactions. Not surprisingly, the larger, more hydrophobic tetracyclic compounds exhibit improved binding and inhibition properties. The increase in binding affinity of these compounds likely results from the formation of a large area for favorable π–π interactions, as well as from the exclusion of water molecules from the hQR2 active site that are not displaced by the smaller bicyclic compounds [86]. Based on the conclusions from the X-ray studies, the compounds 3, 14 perfectly fit the structural requirements for the high-affinity ligands at hQR2.

Recently, the studies on the mechanism of action of afobazole (22), a selective anxiolytic drug that was approved in Russia in 2005 [90–95], identified this drug to be a multifunctional pharmacophore [90]. Initially, afobazole (22) was developed and approved on the basis of in vivo preclinical studies without a clear understan-ding of the molecular mechanism of action. In preclinical and clinical development, afobazole has been shown to decrease neuronal death in vitro in response to oxidative stress and glutamate excitotoxicity and demonstrated anxiolytic, antidepressant, and neuroprotective effects in vivo [90].

Similarly to ambocarb (3), afobazole (22) was recently studied in the radioligand binding Brain Panel at CEREP (www.cerep.fr) and demonstrated preferential binding with the IC50 values at MT3 (0.95 μM), σ1 (5.9 μM), and MT1 (16 μM) receptors, as well as the reverse inhibition of MAO-A at 36 μM [91]. The major active metabolite of afobazole M-11 (23) exhibited exclusive binding at MT3 with IC50 = 0.39 μM [91].
Inhibition of DYRK1A Kinase
The dual-specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A) gene is located within the Down syndrome critical region on chromosome 21. Overexpression of DYRK1A has been proposed to be a significant contributor to the underlying neurodevelopmental abnormalities associated with Down syndrome. Transgenic animals overexpressing DYRK1A show marked cognitive deficits and impairment in hippocampal-dependent memory tasks [96, 97]. Studies in cell culture models and transgenic models of Down syndrome that overexpress DYRK1A implicate the DYRK1A kinase in the generation of both amyloid and tau pathologies associated with early onset Alzheimer’s disease (AD) that is uniformly observed in Down syndrome [98–102]. Recently, several groups have shown that DYRK1A is important for phosphorylation of tau protein on multiple sites in several cellular models [98–100, 102]. These combined observations raise the possibility that DYRK1A may be a critical contributor to tau dysfunction and tau pathology of Alzheimer’s disease and, moreover, that this kinase may be an important therapeutic target for pharmacological interventions seeking to modify the course of tau pathology in AD.
The family of β-carboline alkaloids 24-29 (considered in the subset C, Figs. 3, 4) are naturally occurring compounds in some plant species that affect multiple central nervous system targets, including a high affinity inhibition of DYRK1A kinase activity [103, 104]. This suggests that harmine (24), and possibly other compounds with the β-carboline fragment, could alter tau phosphorylation. Two inhibitors of DYRK1A from the tetrahydroindolo[2,3-c]quinolinone series, ambocarb (3) and compound 14d, have been studied in cell-based assays for inhibition of DYRK1A activity and tau phosphorylation along with the β-carboline alkaloids 24–29 [59, 60, 105].

The results demonstrated that the tested compounds potently reduce the expression of all three phosphorylated forms of tau protein and inhibit the DYRK1A-catalyzed direct phosphorylation of tau protein on serine 396 (Table 2). By assaying several compounds, certain structural criteria that modulate the affinity for inhibition of tau phosphorylation have been identified. Specifically, 9-ethylharmine (29) has one of the highest effects on inhibition of pS396 tau phosphorylation comparing with the N(9)-unsubstituted analogs (compounds 24, 26-28) (Table 2). Thus, introduction of an alkyl substituent at N(7) atom in the compounds 14 may result in enhanced DYRK1A inhibition by the tetrahydroindolo[2,3-c]quinolinones of type 15. Further refinement of this class of compounds could lead to high affinity inhibitors of tau phosphorylation.
Inhibition of Neuronal L-type Calcium Channels
Substantial preclinical and clinical data on the L-type calcium channel blockers support their efficacy for the treatment of various CNS disorders, such as serving as neuroprotectants (stroke/ischemia, traumatic brain injury), antidepressants and mood stabilizers (major depression and bipolar disorders), analgesics (neuropathic pain/opiate potentiation), and anticonvulsants (seizures and epilepsy) [106]. However, hypotensive effects of the classical L-type blockers are dominant due to extremely poor brain distribution that imposes significant limitations of the CNS therapeutic utility of L-type calcium channel blockers. This is not the case for ambocarb (3), which distributes into and out of the brain rapidly with a brain-plasma ratio of > 2.6 that appears to be relatively consistent over the time period of observations, suggesting that it is neither actively transported into nor out of the brain [58–60, 62, 63]. Other compounds in the series, particularly the fluoro-substituted analogs 14b-d,i,k-n, could potentially have even better brain distribution and lower metabolic rate.
Recently, the regulation of mitochondrial calcium cycling has been proposed as a strategy to develop novel triple-target neuroprotective compounds for the treatment of Alzheimer’s disease [107]. It was also demonstrated that Aβ oligomers are strongly associated with increased levels of intracellular Ca2+, and L-type calcium channel blockers can prevent Ca2+ influx and protect MC65 human neuroblastoma cells [108]. The neuroprotective effect of L-type calcium channel blockers against amyloid pathogenesis was confirmed in rodents [109].
Ambocarb (3) and selected compounds 14 were tested in MC65 human neuroblastoma cells, and all compounds greatly increased cell survival at low nM concentrations; whole cell patch-clamp electrophysiology conventional study with ambocarb in rat dorsal root ganglia cells at 150 μM confirmed blockage of L-type vs. P/Q and N-type calcium currents (unpublished).
In vivo Profiling of Tetrahydroindolo[2,3-c]quinolinones
The cognitive-enhancing effects of ambocarb (3) were earlier investigated in preclinical studies in several animal models [34, 39]. Also in preclinical studies, ambocarb (3) was established to be highly neuroprotective in rodent models of traumatic shock and hypoxia [33, 34].
For the potential antipsychotic effects, ambocarb (3) was evaluated by the platforms SmartCube® at PsychoGenics, Inc. (www.psychogenics.com). The platforms combine robotics, computer vision, and bio/cheminformatics technologies to automate the measurement and analysis of the effects of CNS drug candidates on mouse behavior. Advantages of the system include (1) improved accuracy, with elimination of subjectivity in behavioral observations, (2) high content, with measurement of over two thousand features of mouse behavior in a single session, which gives rise to complex drug signatures, and (3) multiple indications, with identification of a drug candidate’s ability to be clinically developed in several CNS drug classes. Ambocarb demonstrated robust anxiolytic, antidepressant, and carbamazepine-like mood-stabilizing dose-differentiated signatures at three doses tested (5, 10, and 20 mg/kg).
Ambocarb (3) and selected compounds 14 were evaluated in vivo and in vitro as anticonvulsants, neuroprotectants and analgesics in the Anticonvulsant Screening Program (ASP) at the National Institute of Neurological Disorders and Stroke (NINDS) [61–63]. Several compounds were effective as (1) anticonvulsants in corneal kindling, picrotoxin, and Frings AUG rodent models, with a broad range of protective indices from 10 to > 120; (2) neuroprotectants in rat brain slices against kainate-induced neurotoxicity; and (3) analgesics in rodent models of acute and/or neuropathic pain [61–63].
Thus, the multifunctional tetrahydroindolo[2,3-c]quinolinone compounds possess a broad range of neuroprotective, cognitive-enhancing, antipsychotic, anticonvulsant, and analgesic in vivo effects, which are compound-specific and dose-dependent.
In summary, testing the hypothesis of “deconvolution” (Fig. 3) on an “old holistic” drug ambocarb resulted in (1) clarification of the multifunctional nature of ambocarb; (2) development of novel analogs of ambocarb, compounds with rebalanced triple-target in vitro profile of the parent pharmacophore; and (3) elucidation of the applicability of the series for the potential treatment of neurodegenerative diseases via triple synergistic and/or additive mechanism of action.
Two possible ways can be considered for rebalancing of the triple action in vitro profile for tetrahydroindolo[2,3-c]-quinolinone compounds: (1) focusing on the high specificity and selectivity of a single selected target for a novel drug candidate, or (2) developing a drug candidate with a balanced triple-action profile. The second approach may have a higher probability of success in developing novel treatments for complex CNS diseases. In addition, based on the identified mechanisms of action, repurposing the clinically validated ambocarb may be considered for the treatment of neurodegenerative and/or psychiatric conditions.
References
J. B. Taylor and D. J. Triggle (editors), Comprehensive Medicinal Chemistry II, Elsevier (2007) (Electronic Edition).
D. J. Abraham and D. P. Rotella (editors), Burger’s Medicinal Chemistry, Drug Discovery and Development, 7th ed., Wiley (2010).
A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, and R. J. K. Taylor (editors), Comprehensive Heterocyclic Chemistry III, Elsevier (2008).
T. Bishop and P. Sham, Analysis of Multifactorial Diseases, Academic Press, New York (2000).
J. Drews, Nat. Rev. Drug Discov., 5, 635 (2006).
B. L. Roth, D. J. Sheffler, and W. K. Kroeze, Nat. Rev. Drug Discov., 3, 353 (2004).
F. Sams-Dodd, Drug Discov. Today, 10, 139 (2005).
F. Sams-Dodd, Drug Discov. Today, 11, 465 (2006).
J. J. Buccafusco, Neurotherapeutics, 6, 4 (2009).
10 . B. Stoica, K. Byrns, and A. I. Faden, Neurotherapeutics, 6, 14 (2009).
R. Vink and A. J. Nimmo, Neurotherapeutics, 6, 28 (2009).
J. Minnerup and W.-R. Schäbitz, Neurotherapeutics, 6, 43 (2009).
M. J. Millan, Neurotherapeutics, 6, 53 (2009).
M. L. Bolognesi, A. Cavalli, and C. Melchiorre, Neurotherapeutics, 6, 152 (2009).
A. Cavalli, M. L. Bolognesi, A. Minarini, M. Rosini, V. Tumiatti, M. Recanatini, and C. Melchiorre, J. Med. Chem., 51, 347 (2008).
K. Solt and S. A. Forman, Curr. Opin. Anaesthesiol., 20, 300 (2007).
R. G. Eckenhoff, Mol. Interv., 1, 258 (2001).
M. T. Bianchi, J. Pathmanathan and S. S. Cash, Med. Hypotheses, 72, 297 (2009).
S. M. Stahl, CNS Spectrums, 14, 71 (2009).
M. T. Bianchi, Med. Hypotheses, 74, 297 (2010).
B. Cusack, A. Nelson, and E. Richelson, Psychopharmacology (Berlin, Ger.), 114, 559 (1994).
S. M. Stahl, CNS Spectrums, 14, 536 (2009).
P. Skolnick, P. Popik, A. Janowsky, B. Beer, and A. S. Lippa, Eur. J. Pharmacol., 461, 99 (2003).
K. Golembiowska, M. Kowalska, and F. P. Bymaster, Synapse, 66, 435 (2012).
P. Tran, P. Skolnick, P. Czobor, N. Y. Huang, M. Bradshaw, A. McKinney, and M. Fava, J. Psychiatr. Res., 46, 64 (2011).
J. T. Metz and P. J. Hajduk, Curr. Opin. Chem. Biol., 14, 498 (2010).
A. L. Hopkins, Nat. Chem. Biol., 4, 682 (2008).
B. Chen, D. Wild, and R. Guha, J. Chem. Inf. Model., 49, 2044 (2009).
R. Morphy, C. Kay, and Z. Rankovic, Drug Discov. Today, 9, 641 (2004).
J. A. Gray and B. L. Roth, Drug Discov. Today: Ther. Strategies, 3, 413 (2006).
O. A. Filz and V. V. Poroikov, Russ. Chem. Rev., 81, 158 (2012).
V. I. Dulenko, V. I. Luk'yanenko, A. V. Kibal'nyi, A. A. Malenko, and Yu. N. Nikolyukin, Khim. Geterotsikl. Soedin., 363 (1985). [Chem. Heterocycl. Compd., 21, 302 (1985).]
I. V. Komissarov, A. V. Titevsky, I. I. Abramets, V. I. Dulenko, Yu. A. Nikolyukin, A. V. Kibal'nyi, T. A. Groshevoi, and G. F. Sukhovoi, RU Pat. Appl. 2064793.
V. I. Dulenko, I. V. Komissarov, Yu. A. Nikolyukin, A. V. Kibal'nyi, A. V. Titevsky, E. Y. Leshchinskaya, UA Pat. Appl. 24293.
I. I. Kut'ko and N. M. Sitchenko, Vrach. Delo, 96 (1995).
D. N. Stephens, H. H. Schneider, W. Kehr, L. H. Jensen, E. Petersen, and T. Honore, Brain Res. Bull., 19, 309 (1987).
C. Braestrup and M. Nielsen, Psychopharmacol. Ser., 11, 1 (1993).
I. V. Komissarov and E. Y. Leshchinskaya, Zh. Akad. Med. Nauk Ukr., 4, 199 (1998).
A. V. Titievsky, O. Railoma, S. A. Nieminen, and M. M. Airaksinen, Pharmacol. Biochem. Behav., 47, 681 (1994).
M. V. Kopanitsa, O. A. Krishtal, and I. V. Komissarov, Clin. Exp. Pharmacol. Physiol., 27, 46 (2000).
I. I. Abramets, I. V. Komissarov, and I. M. Samoilovich, Neurophysiology, 25, 152 (1993).
I. I. Abramets, P. V. Andreev, I. V. Komissarov, and I. M. Samoilovich, Neurophysiology, 26, 301 (1994).
I. V. Komissarov, V. Dulenko, A. T. Dolzhenko, L. Ya. Zin'kovskaya, Yu. A. Nikolyukin, M. Z. Nizharadze, O. G. Abraztsova, A. V. Kibal'nyi, and V. I. Kul'yanenko, Pharm. Chem. J., 19, 187 (1985).
I. V. Komissarov, V. I. Dulenko, A. T. Dolzhenko, Yu. A. Nikolyukin, A. V. Kibal'nyi, L. Ya. Zin'kovskaya, and O. G. Obraztsova, Pharm. Chem. J., 23, 471 (1989).
S. V. Tolkunov, M. N. Kal'nitskii, A. I. Khizhan, S. Yu. Suikov, M. Yu. Zubrtiskii, and V. I. Dulenko, Khim. Geterotsikl. Soedin., 1124 (1995). [Chem. Heterocycl. Compd., 31, 980 (1995).]
A. V. Kibal’nyi, Yu. A. Nikolyukin, and V. I. Dulenko, Fiziol. Akt. Rechovini, 34, 23 (2002).
D. A. Filimonov, V. V. Poroikov, E. I. Karaicheva, R. K. Kazarian, A. P. Budunova, E. M. Mikhailovskii, A. V. Rudnitskikh, L. V. Goncharenko, and Yu. V. Burov, Eksp. Klin. Farmakol., 58, 56 (1995).
Yu. V. Borodina, D. A. Filimonov, and V. V. Poroikov, Pharm. Chem. J., 30, 760 (1996).
D. A. Filimonov and V. V. Poroikov, Ros. Khim. Zh., 50, 66 (2006).
A. Lagunin, D. A. Filimonov, and V. V. Poroikov, Curr. Pharm. Des., 16, 1703 (2010).
R. K. Goel, D. Singh, A. Lagunin, and V. Poroikov, Med. Chem. Res., 20, 1509 (2011).
A. Geronikaki, E. Babaev, J. Daerden, W. Dehaen, D. Filimonov, I. Galaeva, V. Krajneva, A. Lagunin, F. Macaev, G. Molodavkin, V. Poroikov, S. Pogrebnoi, V. Saloutin, A. Stepanchikova, E. Stingaci, N. Tkach, L. Vlad, and T. Voronina, Bioorg. Med. Chem., 12, 6559 (2004).
R. K. Goel, A. Singh, P. S. Naidu, M. P. Mahajah, and S. K. Kulkarni, J. Pharm. Pharm. Sci., 8, 182 (2005).
S. A. Kryzhanovsky, R. M. Salimov, A. A. Lagunin, D. A. Filimonov, T. A. Gloriozova, and V. V. Poroikov, Pharm. Chem. J., 45, 605 (2011).
P. Eleftheriou, A. Geronikaki, D. Hadjipavlou-Litina, P. Vicini, O. Filz, D. Filimonov, V. Poroikov, S. S. Chaudhaery, K. K Roy, and A. Saxena, Eur. J. Med. Chem., 47, 111 (2012).
P. Agarwal and D. B. Searls, Brief. Bioinform., 9, 479 (2008).
I. Shcherbakova and Yu. Nikolyukin, US Pat. Appl. 7820691.
I. Shcherbakova and Yu. Nikolyukin, US Pat. Appl. 20110021776.
I. Shcherbakova and K. Nikolich, US Pat. Appl. 20110136844.
I. Shcherbakova, K. Nikolich and Yu. Nikolyukin, WO Pat. Appl. 2011068990.
I. Shcherbakova and J. P. Stables, Drugs Future, Supl. A/33, P183 (2008).
I. Shcherbakova, Epilepsy Pipeline Update, San Francisco, Feb. 25–26, 2010 (abstract: www.epilepsy.com/pdfs/2010pipeline_program_book.pdf).
I. Shcherbakova, Epilepsy Pipeline Update, San Francisco, Feb. 25–26, 2010 (presentation: http://av.conferencearchives.com/pdfs/100201/PR2.05.pdf).
E. V. Kuznetsov, I. V. Shcherbakova, and A. T. Balaban, Adv. Heterocycl. Chem., 50, 157 (1990).
D. S. Pickering and L. P. Niles, Eur. J. Pharmacol., 175, 71 (1990).
M. L. Dubovich, Trends Pharmacol. Sci., 16, 50 (1995).
E. J. Molinari, P. C. North, and M. L. Dubocovich, Eur. J. Pharmacol., 301, 159 (1996).
P. Paul, C. Lahaye, P. Delagrange, J. P. Nicolas, E. Canet, and J. A. Boutin, J. Pharmacol. Exp. Ther., 290, 334 (1999).
O. Nosejean, M. Ferro, F. Cogé, P. Beauverger, J.-M. Henlin, F. Lefoulon, J.-L. Fauchère, P. Delagrange, E. Canet, and J. A. Boutin, J. Biol. Chem., 275, 31311 (2000).
O. Nosejean, J.-P. Nicolas, F. Klupsch, P. Delagrange, E. Canet, and J. A. Boutin, Biochem. Pharmacol., 61, 1369 (2001).
F. Mailliet, G. Ferry, F. Vella, K. Thiam, P. Delagrange, and J. A. Boutin, FEBS Lett., 578, 116 (2004).
F. Mailliet, G. Ferry, F. Vella, S. Berger, F. Cogé, P. Chomarat, C. Mallet, S.-P. Guénin, G. Guillaumet, M.-C. Viaud-Massuard, S. Yous, P. Delagrange, and J. A. Boutin, Biochem. Pharmacol., 71, 74 (2005).
B. Calamini, B. D. Santarsiero, J. A. Boutin, and A. D. Mesecar, Biochem. J., 413, 81 (2008).
F. Vella, G. Ferry, P. Delagrange, and J. A. Boutin, Biochem. Pharmacol., 71, 1 (2005).
J. A. Boutin, F. Chatelain-Egger, F. Vella, P. Delagrange, and G. Ferry, Chem.–Biol. Interact., 151, 213 (2005).
P. Delagrange and J. A. Boutin, Chronobiol. Int., 23, 413 (2006).
M.-F. Boussard, S. Truche, A. Rousseau-Rojas, S. Briss, S. Descamps, M. Droual, M. Wierzbicki, G. Ferry, V. Audinot, P. Delagrange, and J. A. Boutin, Eur. J. Med. Chem., 41, 306 (2006).
P. Chomarat, F. Cogé, S. Guénin, F. Mailliet, F. Vella, C. Mallet, S. Giraudet, N. Nagel, S. Leonce, G. Ferry, P. Delagrange, and J. A. Boutin, Biochimie, 89, 1264 (2007).
J. A. Boutin, C. Saunier, S.-P. Guenin, S. Berger, N. Moulharat, A. Gohier, P. Delagrange, F. Cogé, and G. Ferry, Arch. Biochem. Biophys., 477, 12 (2008).
J. A. Boutin, E. Marcheteau, P. Hennig, N. Moulharat, S. Berger, P. Delagrange, J.-P. Bouchet, and G. Ferry, J. Pineal Res., 45, 524 (2008).
M. Ettaoussi, B. Péres, F. Klupsch, P. Delagrange, J. A. Boutin, P. Renard, D. H. Caignard, P. Chavatte, P. Berthelot, D. Lesieur, and S. Yous, Bioorg. Med. Chem., 16, 4954 (2008).
R. Jockers, P. Maurice, J. A. Boutin, and P. Delagrange, Br. J. Pharmacol., 154, 1182 (2008).
C. E. Benoit, S. Bastianetto, J. Brouillette, Y. C. Tse, J. A. Boutin, P. Delagrange, T. P. Wong, P. Sarret, and R. Quirion, J. Neurosci., 30, 12690 (2010).
G. Ferry, S. Hecht, S. Berger, N. Moulharat, F. Coge, G. Guillaumet, V. Leclerc, S. Yous, P. Delagrange, and J. A. Boutin, Chem.–Biol. Interact., 186, 103 (2010).
L. Vincent, W. Cohen, P. Delagrange, J. A. Boutin, and O. Nosjean, J. Pineal Res., 48, 222 (2010).
S. D. Peġan, M. Sturdy, G. Ferry, P. Delagrange, J. A. Boutin, and A. D. Mesecar, Protein Sci., 20, 1182 (2011).
M. Antoine, E. Marcheteau, P. Delagrange, G. Ferry, and J. A. Boutin, Int. J. Mass Spectrom., 312, 87 (2012).
T. Hashimoto and M. Nakai, Neurosci. Lett., 502, 10 (2011).
D. P. Zlotos, Curr. Med. Chem., 19, 3532 (2012).
T. A. Zenina, I. V. Gavrish, D. S. Melkumyan, T. S. Seredenina, and S. B. Seredenin, Bull. Exp. Biol. Med., 140, 194 (2005).
S. R. Seredenin and M. V. Voronin, Eksp. Klin. Farmakol., 72, 3 (2009).
S. B. Seredenin and V. A. Kraineva, Eksp. Klin. Farmakol., 72, 24 (2009).
S. B. Seredenin, G. M. Molodavkin, M. V. Voronin, and T. A. Voronina, Eksp. Klin. Farmakol., 72, 21 (2009).
S. B. Seredenin, T. A. Garibova, A. L. Kuznetsova, M. V. Voronin, M. A. Yarkova, and T. A. Voronina, Eksp. Klin. Farmakol., 72, 15 (2009).
S. G. Burchinsky, Liky Ukr., No 4, 48 (2010).
X. Altafaj, M. Dierssen, C. Baamonde, E. Martí, J. Visa, J. Guimerà, M. Oset, J. R. Gonzàlez, J. Flórez, C. Fillat, and X. Estivill, Hum. Mol. Genet., 10, 1915 (2001).
K.-J. Ahn, H. K. Jeong, H.-S. Choi, S.-R. Ryoo, Y. J. Kim, J.-S. Goo, S.-Y. Choi, J.-S. Han, I. Ha, and W.-J. Song, Neurobiol. Dis., 22, 463 (2006).
R. Kimura, K. Kamino, M. Yamamoto, A. Nuripa, T. Kida, H. Kazui, R. Hashimoto, T. Tanaka, T. Kudo, H. Yamagata, Y. Tabara, T. Miki, H. Akatsu, K. Kosaka, E. Funakoshi, K. Nishitomi, G. Sakaguchi, A. Kato, H. Hattori, T. Uema, and M. Takeda, Hum. Mol. Genet., 16, 15 (2007).
J. Park, E. J. Yang, J. H. Yoon, and K. C. Chung, Mol. Cell. Neurosci., 36, 270 (2007).
S.-R. Ryoo, H.-J. Cho, H.-W. Lee, H. K. Jeong, C. Radnaabazar, Y.-S. Kim, M.-J. Kim, M.-Y. Son, H. Seo, S.-H. Chung, and W.-J. Song, J. Neurochem., 104, 1333 (2008).
S.-R. Ryoo, H. K. Jeong, C. Radnaabazar, J.-J. Yoo, H.-J. Cho, H.-W. Lee, I.-S. Kim, Y.-H. Cheon, Y. S. Ahn, S.-H. Chung, and W.-J. Song, J. Biol. Chem., 282, 34850 (2007).
D. O. Azorsa, R. H. Robeson, D. Frost, B. Meechoovet, G. R. Brautigam, C. Dickey, C. Beaudry, G. D. Basu, D. R. Holz, J. A. Hernandez, K. M. Bisanz, L. Gwinn, A. Grover, J. Rogers, E. M. Reiman, M. Hutton, D. A. Stephan, S. Mousses, and T. Dunckley, BMC Genomics, 11, 25 (2010).
J. Bain, L. Plater, M. Elliott, N. Shpiro, C. J. Hastie, H. McLauchlan, I. Klevernic, J. S. Arthur, D. R. Alessi, and P. Cohen, Biochem. J., 408, 297 (2007).
N. Göckler, G. Jofre, C. Papadopoulos, U. Soppa, F. J. Tejedor, and W. Becker, FEBS J., 276, 6324 (2009).
D. Frost, B. Meechoovet, T. Wang, S. Gately, M. Giorgetti, I. Shcherbakova, and T. Dunckley, PLoS ONE, 6, e19264 (2011).
C. Doering, G. Zamponi, in: D. J. Triggle, M. Gopalakrishnan, D. Rampe, W. Zheng (editors), Voltage-Gated Ion Channels as Drug Targets, Wiley-VCH (2006), p. 65.
J.-C. Fernandez-Morales, J.-A. Arranz-Tagarro, E. Calvo-Gallardo, M. Maroto, J.-F. Padín, and A. G. García, ACS Chem. Neurosci., 3, 873 (2012).
T. S. Anekonda, J. F. Quinn, C. Harris, K. Frahler, T. L. Wadsworth, and R. L. Woltjer, Neurobiol. Dis., 41, 62 (2011).
H. Gholamipour-Badie, N. Naderi, F. Khodagholi, F. Shaerzadeh, and F. Motamedi, Behav. Brain Res., 237, 190 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 6–24, January, 2013.
Rights and permissions
About this article
Cite this article
Shcherbakova, I. A Drug Mystery of Heterocycles: Various Molecules for One Target or One Compound for Multiple Targets?. Chem Heterocycl Comp 49, 2–18 (2013). https://doi.org/10.1007/s10593-013-1229-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1229-x