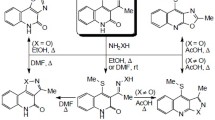
The heterocyclization reaction of pyrazolinylquinolinone and its 4-chlorobenzylidene, 2-pyridylmethylene, pyridylaminomethylene, and 2-pyridylhydrazone derivatives with some active methylene nitriles and acrylonitriles, is described. These cyclization reactions afforded novel heterocyclic systems, such as pyrazolo[3,4-b]pyridines, pyrano[2,3-c]pyrazoles, and pyrazolo[4,3-c]pyridazines, linked to position 3 of 4-hydroxy-1-methylquinolin-2(1H)-one. The effect of the new products on the activity of α-amylase was examined. Some pyrano[2,3-c]pyrazoles revealed significant increase in α-D-glucose production by the enzyme.





Similar content being viewed by others
References
M. Abass and A. Khodairy, Phosphorus, Sulfur, Silicon, Relat. Elem., 186, 287 (2011).
M. Azad, M. A. Munawar, and M. Athar, J. Appl. Sci., 7, 1620 (2007).
O. Jansen, V. Akhmedjanova, L. Angenot, G. Balansard, A. Chariot, E. Ollivier, M. Tits, and M. Frédérich, J. Ethnopharmacol., 105, 241 (2006).
A. Afonso, J. Weinstein, and M. J. Gentles, US Pat. 5382572 (1995). http://www.freepatentsonline.com/5382572.pdf
B. Podeszwa, H. Niedbala, J. Polanski, R. Musiol, D. Tabak, J. Finster, K. Serafin, M. Milczarek, J. Wietrzyk, S. Boryczka, W. Mol, J. Jampilek, J. Dohnal, D. S. Kalinowski, and D. R. Richardson, Bio- org. Med. Chem. Lett., 17, 6138 (2007).
I. V. Ukrainets, S. G. Taran, L. V. Sidorenko, O. V. Gorokhova, A. A. Ogirenko, A. V. Turov, and N. I. Filimonova, Chem. Heterocycl. Comp., 32, 960 (1996). [Khim.Geterotsikl. Siedin., 1113 (1996)].
M. Abass and B. B. Mostafa, Bioorg. Med. Chem., 13, 6133 (2005).
R. Musiol, D. Tabak, H. Niedbala, B. Podeszwa, J. Jampilek, K. Kralova, J. Dohnal, J. Finster, A. Mencel, and J. Polanski, Bioorg. Med. Chem., 16, 4490 (2008).
K. Findeisen and P.-E. Frohberger, US Pat. 3686410 (1972). http://www.freepatentsonline.com/3686410.pdf
M. J. Drysdale, B. W. Dymock, X. Barril-Alonso, P. Workman, L. H. Pearl, C. Prodromou, and E. McDonald, US Pat. 7247734 (2007). http://www.freepatentsonline.com/7247734.pdf
A. Gürsoy, Ş. Demirayak, G. Çapan, K. Erol, and K. Vural, Eur. J. Med. Chem. 35, 359 (2000).
B. Hinz, O. Cheremina, J. Bachmakov, B. Renner, O. Zolk, M. F. Fromm, and K. Brune, FASEB J., 21, 2343 (2007).
R. Pamukcu and G. A. Piazza, US Pat. 6034099 (2000). http://www.freepatentsonline.com/6034099.pdf
D. E. Kuhla, H. F. Campbell, W. L. Studt, and W. C. Faith, US Pat. 4954494 (1990). http://www.freepatentsonline.com/4954494.pdf
S. S. Szucs and K.-J. Gu, US Pat. 6465395 (2002). http://www.freepatentsonline.com/6465395.pdf
S. Kuribayashi, K. Goto, S. Naito, T. Kamataki, and H. Yamazaki, Chem. Res. Toxicol., 22, 323 (2009).
B. M. Lynch, M. A. Khan, H. C. Teo, and F. Pedrotti, Can. J. Chem., 66, 420 (1988).
S. Saggar, J. T. Sisko, T. J. Tucker, R. M. Tynebor, D. Su, and N. J. Anthony, US Pat. 2007/021442 (2007). http://www.freepatentsonline.com/20070021442.pdf
Y. D. Wang, E. Honores, B. Wu, S. Johnson, D. Powell, M. Miranda, J. P. McGinnis, C. Discafani, S. K. Rabindran, W. Cheng, and G. Krishnamurthy, Bioorg. Med. Chem., 17, 2091 (2009).
N. R. Mohamed, N. Y. Khaireldin, A. F. Fahmy, and A. A. El-Sayed, Der Pharma Chemica, 2, 400 (2010). http://derpharmachemica.com/vol2-iss1/DPC-2010-2-1-400-417.pdf
B. A. Meiners and A. I. Salama, Eur. J. Pharmacol., 78, 315 (1982).
S. C. Kuo, L. J. Huang, and H. Nakamura, J. Med. Chem., 27, 539 (1984).
N. Foloppe, L. M. Fisher, R. Howes, A. Potter, A. G. S. Robertson, and A. E. Surgenor, Bioorg. Med. Chem., 14, 4792 (2006).
J. Witherington, V. Bordas, A. Gaiba, N. S. Garton, A. Naylor, A. D. Rawlings, B. P. Slingsby, D. G. Smith, A. K. Takle, and R. W. Ward, Bioorg. Med. Chem. Lett., 13, 3055 (2003).
J. W. Myatt, M. P. Healy, G. S. Bravi, A. Billinton, C. N. Johnson, K. L. Matthews, K. S. Jandu, W. Meng, A. Hersey, D. G. Livermore, C. B. Douault, J. Witherington, R. A. Bit, J. E. Rowedder, J. D. Brown, and N. M. Clayton, Bioorg. Med. Chem. Lett., 20, 4683 (2010).
Z. K. Sweeney, S. F. Harris, N. Arora, H. Javanbakht, Y. Li, J. Fretland, J. P. Davidson, J. R. Billedeau, S. K. Gleason, D. Hirschfeld, J. J. Kennedy-Smith, T. Mirzadegan, R. Roetz, M. Smith, S. Sperry, J. M. Suh, J. Wu, S. Tsing, A. G. Villaseňor, A. Paul, G. Su, G. Heilek, J. Q. Hang, A. S. Zhou, J. A. Jernelius, F.-J. Zhang, and K. Klumpp, J. Med. Chem., 51, 7449 (2008).
M. F. Braña, M. Cacho, M. L. García, E. P. Mayoral, B. López, B. de Pascual-Teresa, A. Ramos, N. Acero, F. Llinares, D. Muñoz-Mingarro, O. Lozach, and L. Meijer, J. Med. Chem., 48 , 6843 (2005).
A. Khodairy, Synth. Commun., 31, 2697 (2001).
A. Khodairy, J. Chinese Chem. Soc., 54, 93 (2007).
M. Abass and E. S. Othman, Synth. Commun., 31, 3361 (2001).
M. Abass, E. S. Othman, and A. Hassan, Synth. Commun., 37, 607 (2007).
M. Abass, Phosphorus, Sulfur, Silicon, Relat. Elem., 182, 735 (2007).
M. M. Ismail, Chem. Papers, 55, 242 (2001).
A. Rahmati, Tetrahedron Lett., 51, 2967 (2010).
S. K. Panja, S. Maiti, S. Banerjee, and C. Bandyopadhyay, Synlett , 1909 (2010).
J. O. Subbotina, W. M. F. Fabian, E. V. Tarasov, N. N. Volkova, and V. A. Bakulev, Eur. J. Org. Chem., 2914 (2005).
M. H. Elnagdi, F. M. Abdelrazek, N. S. Ibrahim, and A. W. Erian, Tetrahedron, 45, 3597 (1989).
P. Trinder, Ann. Clin. Biochem., 6, 24 (1969).
G. Siest, J. Henny, and F. Schiele (editors), Interprétation des examens de laboratoire. Valeurs de reference et variations biologiques, Karger, Bâle, 1981, p. 206.
Author information
Authors and Affiliations
Corresponding author
Additional information
* For Communication 14, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 736–749, May, 2011.
Rights and permissions
About this article
Cite this article
Khodairy, A., Abass, M. Substituted quinolinones 15*. Preparation and enzymatic activity of some pyrazoloazines linked to the 4-hydroxy-1-methyl- quinolin-2(1H)-one moiety. Chem Heterocycl Comp 47, 611–621 (2011). https://doi.org/10.1007/s10593-011-0806-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0806-0