Abstract
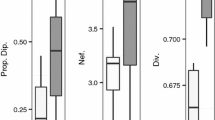
Orchid bees (Hymenoptera, Apidae, Euglossini) are important pollinators of many plant families in Neotropical forests, habitats that have become increasingly degraded and fragmented by agricultural practices. To understand the extent to which loss of natural habitat and isolation has affected the genetic diversity and diploid male production (DMP) of two orchid bee species, Euglossa dilemma and Euglossa viridissima, we collected and genotyped 1686 males at five microsatellite loci and tested for differences in allelic richness, heterozygosity and DMP across three different types of land use (natural, agricultural and urban) and between mainland and island populations in the Yucatan Peninsula of Mexico. We also investigated the impact of land use and geographic isolation on gene flow. Euglossa dilemma and E. viridissima seemed to be particularly resilient to loss of natural habitat; in locations with human impact, we did not find reduced genetic diversity, and populations generally showed very little population genetic structure. Only on islands did E. dilemma show significantly reduced genetic diversity. Even after accounting for putative null alleles, DMP was very low (0.2–1.3%) across all sampling sites, including on islands. We therefore suggest that DMP is an insensitive measure of inbreeding and population decline in our two study species.


Similar content being viewed by others
References
Anonymous (2010) http://comey.yucatan.gob.mx/marco_files/II.6_Desarrollo_urbano_y_OT.pdf.
Barbier EB (2004) Agricultural expansion, resource booms and growth in Latin America: implication for long-run economic development. World Dev 32:137–157
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. doi:10.18637/jss.v067.i01
Beye M, Hasselmann M, Fondrk MK, Page RE Jr, Omholt SW (2003) The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114:419–429
Boff S, Soro A, Paxton R, Alves-dos-Santos I (2014) Island isolation reduces genetic diversity and connectivity but does not significantly elevate diploid male production in a neotropical orchid bee. Conserv Genet 15:1123–1135. doi:10.1007/s10592-014-0605-0
Bohonak A (1999) Dispersal, gene flow, and population structure. Q Rev Biol 74:21–45
Brand P, Ramirez S, Leese F, Quezada-Euan J, Tollrian R, Eltz T (2015) Rapid evolution of chemosensory receptor genes in a pair of sibling species of orchid bees (Apidae: Euglossini). BMC Evol Biol 15:176
Brown MJF, Paxton RJ (2009) The conservation of bees: a global perspective. Apidologie 40:410–416. doi:10.1051/apido/2009019
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 108:662–667
Cerântola NM, Oi CA, Cervini M, Del Lama A (2011) Genetic differentiation of urban populations of Euglossa cordata from the state of São Paulo, Brazil. Apidologie 42:214–222. doi:10.1051/apido/2010055
Cincotta RP, Engelman R (2000) Nature’s place: human population and the future of biological diversity. Population Action International, Washington, DC
Cocom Pech M, May-Itzá WdJ, Medina Medina L, Quezada-Euán J (2008) Sociality in Euglossa (Euglossa) viridissima Friese (Hymenoptera, Apidae, Euglossini). Insectes Soc 55:428–433. doi:10.1007/s00040-008-1023-4
Cook JM (1993) Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71:421–435
Cook JM, Crozier RH (1995) Sex determination and population biology in the hymenoptera. Trends Ecol Evol 10:281–286
Coulon A, Fitzpatrick JW, Bowman R, Stith BM, Makarewich CA, Stenzler LM, Lovette IJ (2008) Congruent population structure inferred from dispersal behaviour and intensive genetic surveys of the threatened Florida scrub-jay (Aphelocoma cœrulescens). Mol Ecol 17:1685–1701. doi:10.1111/j.1365-294X.2008.03705.x
Crozier RH (1971) Heterozygosity and sex determination in haplo-diploidy. Am Nat 105:399–412. doi:10.2307/2459509
Crozier RH, Pamilo P (1996) Evolution of social insect colonies. Sex allocation and Kin selection. Oxford University Press, Oxford
Darvill B, Ellis J, Lye G, Goulson D (2006) Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Mol Ecol 15:601–611. doi:10.1111/j.1365-294x.2006.02797.x
Davis ES, Murray TE, Fitzpatrick U, Brown MJF, Paxton RJ (2010) Landscape effects on extremely fragmented populations of a rare solitary bee, Colletes floralis. Mol Ecol 19:4922–4935. doi:10.1111/j.1365-294X.2010.04868.x
de Boer J, Groenen M, Pannebakker B, Beukeboom L, Kraus R (2015) Population-level consequences of complementary sex determination in a solitary parasitoid. BMC Evol Biol 15:98
Dieringer D, Schlötterer C (2003) microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169. doi:10.1046/j.1471-8286.2003.00351.x
Dressler RL (1982) Biology of the orchid bees (Euglossini). Annu Rev Ecol Syst 13:373–394. doi:10.1146/annurev.es.13.110182.002105
Dufresnes C, Perrin N (2015) Effect of biogeographic history on population vulnerability in European amphibians. Conserv Biol 29:1235–1241. doi:10.1111/cobi.12490
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Ellis J, Knight M, Darvill B, Goulson D (2006) Extremely low effective population sizes, genetic structuring and reduced genetic diversity in a threatened bumblebee species, Bombus sylvarum (Hymenoptera: Apidae). Mol Ecol 15:4375–4386
Eltz T et al (2008) An olfactory shift is associated with male perfume differentiation and species divergence in orchid bees. Curr Biol 18:1844–1848. doi:10.1016/j.cub.2008.10.049
Eltz T, Fritzsch F, Pech JR, Zimmermann Y, RamÍRez SR, Quezada-Euan JJG, BembÉ B (2011) Characterization of the orchid bee Euglossa viridissima (Apidae: Euglossini) and a novel cryptic sibling species, by morphological, chemical, and genetic characters. Zool J Linn Soc 163:1064–1076. doi:10.1111/j.1096-3642.2011.00740.x
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142. doi:10.1017/s1464793105006949
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol S 34:487–515. doi:10.1146/annurev.ecolsys.34.011802.132419
Farias IP, Santos WG, Gordo M, Hrbek T (2015) Effects of forest fragmentation on genetic diversity of the critically endangered primate, the pied yamarin (Saguinus bicolor): implications for conservation. J Hered 106:512–521. doi:10.1093/jhered/esv048
Francisco FO, Santiago LR, Mizusawa YM, Oldroyd BP, Arias MC (2015) Genetic structure of island and mainland populations of a Neotropical bumble bee species. J Insect Conserv 20:383–394. doi:10.1007/s10841-016-9872-z
Frankham R (1995) Conservation genetics. Annu Rev Genet 29:305–327. doi:10.1146/annurev.ge.29.120195.001513
Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity 78:311–327
Frankham R (2003) Genetics and conservation biology. CR Biol 326:22–29. doi:10.1016/S1631-0691(03)00023-4
Franzén M, Nilsson SG (2010) Both population size and patch quality affect local extinctions and colonizations. P R Soc Lond B 277:79–85
Galbusera P, Githiru M, Lens L, Matthysen E (2004) Genetic equilibrium despite habitat fragmentation in an Afrotropical bird. Mol Ecol 13:1409–1421. doi:10.1111/j.1365-294X.2004.02175.x
Gempe T, Beye M (2011) Function and evolution of sex determination mechanisms, genes and pathways in insects. BioEssays 33:52–60. doi:10.1002/bies.201000043
Giangarelli D, Freiria G, Ferreira D, Aguiar WA, Penha RES et al (2015) Orchid bees: a new assessment on the rarity of diploid males in populations of this group of Neotropical pollinators. Apidologie 46:606–617
Gilpin ME, Soulé ME (1986) Minimum viable populations: process of species extinction. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, pp 19–34
Gómez-Pompa A, Kaus A (1999) From pre-Hispanic to future conservation alternatives: lessons from Mexico. Proc Natl Acad Sci USA 96:5982–5986
Gomez-Pompa A, Vazquez-Yanes C, Guevara S (1972) The tropical rain forest: a nonrenewable resource. Science 177:762–765
Goulson D, Kaden JC, Lepais O, Lye GC, Darvill B (2011) Population structure, dispersal and colonization history of the garden bumblebee Bombus hortorum in the Western Isles of Scotland. Conserv Genet 12:867–879. doi:10.1007/s10592-011-0190-4
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957. doi:10.1126/science.1255957
Hanski I (2011) Habitat loss, the dynamics of biodiversity and a perspective on conservation. AMBIO 40:248–255. doi:10.1007/s13280-011-0147-3
Harpur BA, Sobhani M, Zayed A (2013) A review of the consequences of complementary sex determination and diploid male production on mating failures in the Hymenoptera. Entom Exp Appl 146:156–164. doi:10.1111/j.1570-7458.2012.01306.x
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Heimpel GE, de Boer JG (2008) Sex determination in the Hymenoptera. Annu Rev Entomol 53:209–230
Hoban S, Arntzen JA, Bruford MW, Godoy JA, Rus Hoelzel A et al (2014) Comparative evaluation of potential indicators and temporal sampling protocols for monitoring genetic erosion. Evol Appl 7:984–998
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363
Husemann M, Cousseau L, Callens T, Matthysen E, Vangestel C, Hallmann C, Lens L (2015) Post-fragmentation population structure in a cooperative breeding Afrotropical cloud forest bird: emergence of a source-sink population network. Mol Ecol 24:1172–1187. doi:10.1111/mec.13105
Jackson ND, Fahrig L (2014) Landscape context affects genetic diversity at a much larger spatial extent than population abundance. Ecology 95:871–881. doi:10.1890/13-0388.1
Jaffé R, Castilla A, Pope N, Imperatriz-Fonseca VL, Metzger JP, Arias MC, Jha S (2015) Landscape genetics of a tropical rescue pollinator. Conserv Genet 17:267–278. doi:10.1007/s10592-015-0779-0
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205. doi:10.1126/science.171.3967.203
Jost L (2008) GST and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Keller D, van Strien MJ, Herrmann M, Bolliger J, Edwards PJ, Ghazoul J, Holderegger R (2013) Is functional connectivity in common grasshopper species affected by fragmentation in an agricultural landscape? Agr Ecosyst Environ 175:39–46. doi:10.1016/j.agee.2013.05.006
Koh I, Lonsdorf EV, Williams NM, Brittain C, Isaacs R, Gibbs J, Ricketts TH (2016) Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc Natl Acad Sci USA 113:140–145. doi:10.1073/pnas.1517685113
Landaverde-González P, Enríquez E, Ariza MA, Murray T, Paxton RJ, Husemann M (2016) Habitat fragmentation and the population genetics of the native bee species Partamona bilineata (Hymenoptera: Apidae: Meliponini) in the cloud forest of Guatemala. In review, this special issue
Landguth EL, Cushman SA, Schwartz MK, McKelvey KS, Murphy M, Luikart G (2010) Quantifying the lag time to detect barriers in landscape genetics. Mol Ecol 19:4179–4191. doi:10.1111/j.1365-294X.2010.04808.x
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116. doi:10.1016/j.tree.2013.12.001
Lechner S, Ferretti L, Schöning C, Kinuthia W, Willemsen D, Hasselmann M (2014) Nucleotide variability at its limit? Insights into the number and evolutionary dynamics of the sex-determining specificities of the honey bee Apis mellifera. Mol Biol Evol 31:272–287. doi:10.1093/molbev/mst207
Lewis SL, Edwards DP, Galbraith D (2015) Increasing human dominance of tropical forests. Science 349:827–832
López-Uribe MM, Almanza MT, Ordoñez M (2007) Diploid male frequencies in Colombian populations of euglossine bees. Biotropica 39:660–662. doi:10.1111/j.1744-7429.2007.00287.x
Lozier JD (2014) Revisiting comparisons of genetic diversity in stable and declining species: assessing genome-wide polymorphism in North American bumble bees using RAD sequencing. Mol Ecol 23:788–801. doi:10.1111/mec.12636
Lozier JD, Strange JP, Stewart IJ, Cameron SA (2011) Patterns of range-wide genetic variation in six North American bumble bee (Apidae: Bombus) species. Mol Ecol 20:4870–4888. doi:10.1111/j.1365-294X.2011.05314.x
Maebe K, Meeus I, Ganne M, De Meulemeester T, Biesmeijer K, Smagghe G (2015) Microsatellite analysis of museum specimens reveals historical differences in genetic diversity between declining and more stable Bombus species. PLoS ONE 10:e0127870. doi:10.1371/journal.pone.0127870
Martínez-Cruz B, Godoy JA, Negro JJ (2007) Population fragmentation leads to spatial and temporal genetic structure in the endangered Spanish imperial eagle. Mol Ecol 16:477–486. doi:10.1111/j.1365-294X.2007.03147.x
Matschiner M, Salzburger W (2009) TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25:1982–1983. doi:10.1093/bioinformatics/btp303
May-Itzá W, Medina Medina LA, Medina S, Paxton RJ, Quezada-Euán JJG (2014) Seasonal nest characteristics of a facultatively social orchid bee, Euglossa viridissima, in the Yucatan Peninsula, Mexico. Insectes Soc 61:183–190. doi:10.1007/s00040-014-0342-x
Meirmans PG, Hedrick PW (2011) Assessing population structure: FST and related measures. Mol Ecol Resour 11:5–18. doi:10.1111/j.1755-0998.2010.02927.x
Meirmans PG, Van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi:10.1111/j.2041-210x.2012.00261.x
Napolitano C, Díaz D, Sanderson J, Johnson WE, Ritland K, Ritland CE, Poulin E (2015) Reduced genetic diversity and increased dispersal in guigna (Leopardus guigna) in Chilean fragmented landscapes. J Hered 106:522–536. doi:10.1093/jhered/esv025
Nei M (1977) F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet 41:225–233. doi:10.1111/j.1469-1809.1977.tb01918.x
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nemésio A, Santos LM, Vasconcelos HL (2015) Long-term ecology of orchid bees in an urban forest remnant. Apidologie 46:359–368. doi:10.1007/s13592-014-0328-8
Oomen RA, Reudink MW, Nocera JJ, Somers CM, Green MC, Kyle CJ (2011) Mitochondrial evidence for panmixia despite perceived barriers to gene flow in a widely distributed waterbird. J Hered 102:584–592. doi:10.1093/jhered/esr055
Ortego J, Aguirre M, Cordero PJ (2010) Population genetics of Mioscirtus wagneri, a grasshopper showing a highly fragmented distribution. Mol Ecol 19:472–483. doi:10.1111/j.1365-294X.2009.04512.x
Paxton RJ, Thorén PA, Gyllenstrand N, Tengö J (2000) Microsatellite DNA analysis reveals low diploid male production in a communal bee with inbreeding. Biol J Linn Soc 69:483–502. doi:10.1111/j.1095-8312.2000.tb01220.x
Paxton RJ, Zobel M, Steiner J, Zillikens A (2009) Microsatellite loci for Euglossa annectans (Hymenoptera: Apidae) and their variability in other orchid bees. Mol Ecol Resour 9:1221–1223
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Pellet J, Fleishman E, Dobkin DS, Gander A, Murphy DD (2007) An empirical evaluation of the area and isolation paradigm of metapopulation dynamics. Biol Conserv 136:483–495. doi:10.1016/j.biocon.2006.12.020
Phillipsen IC, Lytle DA (2013) Aquatic insects in a sea of desert: population genetic structure is shaped by limited dispersal in a naturally fragmented landscape. Ecography 36:731–743. doi:10.1111/j.1600-0587.2012.00002.x
Pokorny T, Loose D, Dyker G, Quezada-Euán JJ, Eltz T (2015) Dispersal ability of male orchid bees and direct evidence for long-range flights. Apidologie 46:224–237. doi:10.1007/s13592-014-0317-y
Rosa JF, Ramalho M, Arias MC (2016) Functional connectivity and genetic diversity of Eulaema atleticana (Apidae, Euglossina) in the Brazilian Atlantic Forest Corridor: assessment of gene flow. Biotropica 48:509–517. doi:10.1111/btp.12321
Ross KG, Vargo EL, Keller L, Trager JC (1993) Effect of a founder event on variation in the genetic sex-determining system of the fire ant Solenopsis invicta. Genetics 135:843–854
Roubik DW, Hanson PE (2004) Orchid bees of tropical America. Instituto Nacional de Biodiversidad, Costa Rica
Roubik DW, Weight LA, Bonilla MA (1996) Population genetics, diploid males and limits to social evolution of euglossine bees. Evolution 50:931–935. doi:10.2307/2410866
Rueda X (2010) Understanding deforestation in the southern Yucatán: insights from a sub-regional, multi-temporal analysis. Reg Environ Change 10:175–189. doi:10.1007/s10113-010-0115-7
Ruf D, Dorn S, Mazzi D (2013) Unexpectedly low frequencies of diploid males in an inbreeding parasitoid with complementary sex determination. Biol J Linn Soc 108:79–86. doi:10.1111/j.1095-8312.2012.01976.x
Scheper J et al (2014) Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. PNAS 111:17552–17557. doi:10.1073/pnas.1412973111
Schmieder S, Colinet D, Poirié M (2012) Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants. Nat Commun 3:895
Silveira G, Freitas R, Tosta TA, Rabelo L, Gaglianone M, Augusto S (2015) The orchid bee fauna in the Brazilian savanna: do forest formations contribute to higher species diversity?. Apidologie 46:197–208
Soro A, Field J, Bridge C, Cardinal SC, Paxton RJ (2010) Genetic differentiation across the social transition in a socially polymorphic sweat bee, Halictus rubicundus. Mol Ecol 19:3351–3363
Souza RO, Cervini M, Del Lama A, Paxton RJ (2007) Microsatellite loci for euglossine bees (Hymenoptera: Apidae). Mol Ecol Resour 7:1352–1356
Souza RO et al (2010) Conservation genetics of neotropical pollinators revisited: microsatellite analysis suggests that diploid males are rare in orchid bees. Evolution 64:3318–3326. doi:10.1111/j.1558-5646.2010.01052.x
Suni SS (2016) Population genetics of Euglossa imperialis reveals low genetic diversity and restricted dispersal over a fragmented area. In review, this special issue
Szabo BJ, Ward WC, Weidie AE, Brady MJ et al (1978) Age and magnitude of the late Pleistocene sea-level rise on the eastern Yucatan Peninsula. Geology 6:713–715
Takahashi NC, Peruquetti RC, Del Lama MA, Campos LAdO (2001) A reanalysis of diploid male frequencies in euglossine bees (Hymenoptera: Apidae). Evolution 55:1897–1899. doi:10.1111/j.0014-3820.2001.tb00839.x
Turner BL II, Villar SC, Foster D, Geoghegan J, Keys E et al (2001) Deforestation in the southern Yucatán peninsular region: an integrative approach. Forest Ecol Manag 154:353–370
Tzika AC et al (2008) Population genetics of Galápagos land iguana (genus Conolophus) remnant populations. Mol Ecol 17:4943–4952. doi:10.1111/j.1365-294X.2008.03967.x
van de Zande L, Verhulst EC (2014) Genomic imprinting and maternal effect genes in haplodiploid sex determination. Sex Dev 8:74–82
van Wilgenburg E, Driessen G, Beukeboom L (2006) Single locus complementary sex determination in Hymenoptera: an “unintelligent” design? Front Zool 3:1–15
Vanbergen AJ, Initiative IP (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ. doi:10.1890/120126
Verhulst EC, Beukeboom LW, van de Zande L (2010) Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 328:620–623
Villanueva-Gutierrez R, Quesada-Euan J, Elzt T (2013) Pollen diets of two sibling orchid bee species, Euglossa, in Yucatán, southern Mexico. Apidologie 44:440–446
Wang IJ (2013) Examining the full effects of landscape heterogeneity on spatial genetic variation: a multiple matrix regression approach for quantifying geographic and ecological isolation. Evolution 67:3403–3411
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Yokoyama S, Nei M (1979) Population dynamics of sex-determining alleles in honey bees and self-incompatibility alleles in plants. Genetics 91:609–626
Zayed A (2004) Effective population size in Hymenoptera with complimentary sex determination. Heredity 93:627–630
Zayed A (2009) Bee genetics and conservation. Apidologie 40:237–262
Zayed A, Packer L (2005) Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proc Natl Acad Sci USA 102:10742–10746
Zayed A, Roubik DW, Packer L (2004) Use of diploid male frequency data as an indicator of pollinator decline. P R Soc Lond B 271:S9–S12. doi:10.1098/rsbl.2003.0109
Zimmermann Y, Schorkopf DLP, Moritz RFA, Pemberton RW, Quezada-Euan JJG, Eltz T (2011) Population genetic structure of orchid bees (Euglossini) in anthropogenically altered landscapes. Conserv Genet 12:1183–1194. doi:10.1007/s10592-011-0221-1
Acknowledgements
We thank the editors and referees for comments that helped improve the manuscript, Ramirez Pech, Rubén Medina and Tony Gonzalez for collecting the male individuals used in this study and Petra Leibe and Rita Radzeviciute for their assistance in the laboratory. We thank CONACyT-EU Project FONCICyT 94293 (Mutualismos y abejas en paisajes tropicales) for funding.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soro, A., Quezada-Euan, J.J.G., Theodorou, P. et al. The population genetics of two orchid bees suggests high dispersal, low diploid male production and only an effect of island isolation in lowering genetic diversity. Conserv Genet 18, 607–619 (2017). https://doi.org/10.1007/s10592-016-0912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0912-8