Abstract
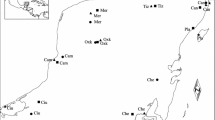
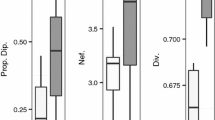
There is concern about the worldwide decline of bees, in which genetic factors may play a role. As populations of these haplodiploid insects suffer habitat fragmentation and subsequent isolation, theory predicts an increase in inbreeding and a concomitant increase in inviable or sterile diploid males, a product of reduced allelic diversity at the sex determining locus, which could lead to a diploid male extinction vortex. To test this idea, we genotyped 1,245 males of one orchid bee, Euglossa cordata, a species with low diploid male production on the mainland. We genotyped bees from the Brazilian mainland and three islands using five highly variable microsatellite loci. Allelic richness was lowest on the most remote island 38 km from the mainland and, though the degree of differentiation across localities was modest (global FST = 0.034; global \({\text{G}}_{\text{ST}}^{'} = 0. 1 5 3\), both P < 0.001) and isolation by distance was weak (Mantel test, r = 0.614, P = 0.056), sea was revealed to be a significant barrier to inferred historic gene flow (partial Mantel test of distance over sea, r = 0.831, P = 0.003). Only seven males were diploid (mean diploid male production, DMP, 0.6 %). Though the proportion of diploid males was highest on the most remote island (1.3 %), differences in DMP across study areas were statistically non-significant. Thus island isolation leads to reduced genetic variation at putatively neutral microsatellite loci, but E. cordata nevertheless seems to maintain allelic diversity at the sex locus, possibly because of sufficient gene flow, or because it is a locus under balancing selection, or because of the joint action of these two evolutionary forces: migration and selection. These and other bee species may be able to maintain sufficient variability at the sex locus to avoid entering the DM extinction vortex, even on relatively isolated islands or habitat fragments.


Similar content being viewed by others
References
Allendorf FW, Luikart G (2007) Conservation and the genetics of populations. Blackwell, Malden
Ângelo S (1989) Ilhas do Litoral Paulista, Série documentos. Secretaria do Meio Ambiente (SMA), São Paulo
Ayasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the Order Hymenoptera. Annu Rev Entomol 46:31–78
Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW (2003) The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114:419–429
Bonin A, Bellemain E, Bronken Eidesen P, Pompanon F, Brochmann C, Taberlet P (2004) How to track and assess genotyping errors in population genetics studies. Mol Ecol 13:3261–3273
Brook BW, Tonkyn DW, O`Grady JJ, Frankham R (2002) Contribution of inbreeding to extinction risk in threatened species. Conserv Ecol 6:16. http://www.consecol.org/vol6/iss1/art16. Accessed 27 Jan 2013
Brown MJF, Paxton RJ (2009) The conservation of bees: a global perspective. Apidologie 40:410–416
Cardinale BJ, Duffy E, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Lagauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67
Cerântola NCM, Oi CA, Cervini M, Del Lama MA (2010) Genetic differentiation of urban populations of Euglossa cordata from the state of São Paulo, Brazil. Apidologie 42:214–222
Cook JM (1993) Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71:421–435
Cook JM, Crozier RH (1995) Sex determination and population biology in the Hymenoptera. Trends Ecol Evol 10:281–286
Cordeiro GD, Boff S, Caetano TA, Fernandes PC, Alves-dos-Santos I (2013) Euglossine bees (Apidae) in Atlantic forest areas of São Paulo State, southeastern Brazil. Apidologie 44:254–267
Cowan DP, Stahlhut JK (2004) Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination. PNAS 101:10374–10379
Crozier RH, Pamilo P (1996) Sex allocation and kin selection. Evolution of social insect colonies. Oxford University Press, Oxford
Darvill B, Ellis JS, Lye GC, Goulson D (2006) Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Mol Ecol 15:601–611
Darvill B, O’Connor S, Lye GC, Waters J, Lepais O, Goulson D (2010) Cryptic differences in dispersal lead to differential sensitivity to habitat fragmentation in two bumblebee species. Mol Ecol 19:53–63
Davis ES, Murray TE, Fitzpatrick Ú, Brown MJF, Paxton RJ (2010) Landscape effects on extremely fragmented populations of a rare solitary bee, Colletes floralis. Mol Ecol 19:4922–4935
de Boer JG, Ode PJ, Rendahl AK, Vet LEM, Whitfield JB, Heimpel GE (2008) Experimental support for multiple-locus complementary sex determination in the parasitoid Cotesia vestalis. Genetics 180:1525–1535
Dennis RLH, Dapporto L, Shreeve TG, John E, Coutsis JG, Kudrna O, Saarinen K, Ryrholm N, (Bob) Williams WR (2008) Butterflies of European islands: the implications of the geography and ecology of rarity and endemicity for conservation. J Insect Conserv 12:205–236
Dieringer D, Schlötterer C (2003) Microsatellite Analyser (MSA 4.05): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Dobson A, Lodge D, Alder J, Cumming GS, Keymer J, McGlade J, Mooney H, Risak JA, Sala O, Wolters V, Wall D, Winfree R, Xenopoulos MA (2006) Habitat loss, trophic collapse, and the decline of ecosystem service. Ecology 87:1915–1924
Dressler RL (1982) Biology of the orchid bees (Euglossini). Annu Rev Ecol Syst 13:373–394
Elias J, Dorn S, Mazzi D (2010) No evidence for increased extinction proneness with decreasing effective population size in a parasitoid with complementary sex determination and fertile diploid males. BMC Evol Biol 10:366
Frankham R (1995) Conservation genetics. Annu Rev Genet 29:305–327
Frankham R (1997) Do island population have less genetic variation than mainland populations? Heredity 78:311–327
Frankham R (1998) Inbreeding and extinction: island populations. Conserv Biol 12:665–675
Frankham R (2003) Genetics and conservation biology. C R Biologies 326:S22–S29
Frankham R, Ballou JD, Briscoe DA (2004) A primer of conservation genetics. Cambridge University Press, Cambridge
Frankham R, Ballou JD, Eldridge MDB, Robert CL, Ralls K, Dudash MR, Fenster CB (2011) Predicting the probability of outbreeding depression. Conserv Biol 25:465–475
Freiria GA, Bombarda Ruim J, Souza RF, Sofia SH (2012) Population structure and genetic diversity of the orchid bee Eufriesea violacea (Hymenoptera, Apidae, Euglossini) from Atlantic Forest remnants in southern and southeastern Brazil. Apidologie 43:392–402
Gallai N, Salles J-M, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted to pollinator decline. Ecol Econ 68:810–821
Garófalo CA (1985) Social structure of Euglossa cordata nests (Hymenoptera: Apidae: Euglossini). Entomol Gen 11:77–83
Gerlach J (2008) Preliminary conservation status and needs of an oceanic island fauna: the case of Seychelles insects. J Insect Conserv 12:293–305
Goulson D, Kaden JC, Lepais O, Lye GC, Darvill B (2011) Population structure, dispersal and colonization history of the garden bumblebee Bombus hortorum in the Western Isles of Scotland. Conserv Genet 12:867–879
Harpur BA, Sobhani M, Zayed A (2013) A review of the consequences of complementary sex determination and diploid male production on mating failures in the Hymenoptera. Entomol Exp Appl 146:156–164
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Heimpel GE, de Boer JG (2008) Sex determination in the Hymenoptera. Annu Rev Entomol 53:209–230
Hein S, Poethke HJ, Dorn S (2009) What stops the ‘diploid male vortex’? A simulation study for species with single locus complementary sex determination. Ecol Model 220:1663–1669
IBM Corporation (2010) IBM SPSS Statistics for Windows, Version 19.0., Released 2010. IBM Corporation, Armonk
Jansen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205
Jansen DH (1981) Reduction in Euglossine bee species richness on Isla del Cano, a Costa Rican offshore island. Biotropica 13:238–240
Jordan MA, Snell HL (2008) Historical fragmentation of islands and genetic drift in populations of Galápagos lava lizards (Microlophus albemarlensis complex). Mol Ecol 17:1224–1237
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Evol Syst 29:83–112
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313
López-Uribe MM, Almanza MT, Ordóñez M (2007) Diploid male frequencies in Colombian population of Euglossini bees. Biotropica 39:660–662
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
McNeely JA, Miller KR, Reid WV, Mittermeier RA, Werner TB (1990) Conserving the World’s biological diversity. IUCN, World Resources Institute, Conservation International, WWF-US and the World Bank, Washington
Meirmans PG, Van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
New TR (2008) Insect conservation on islands: setting the scene and defining the needs. J Insect Conserv 12:197–204
O’Grady JJ, Reed DH, Brook BW, Frankham R (2008) Extinction risk scales better to generations than years. Anim Conserv 11:442–451
Paxton RJ (2005) Male mating behavior and mating systems of bees: an overview. Apidologie 36:145–156
Paxton RJ, Thorén PA, Gyllenstrand N (2000) Microsatellite DNA analysis reveals low diploid male production in a communal bee with inbreeding. Biol J Linnean Soc 69:483–502
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Petit RJ, Deguilloux M-F, Chat J, Grivet D, Garnier-Géré P, Vendramin GG (2005) Standardizing for microsatellite length in comparisons of genetic diversity. Mol Ecol 14:885–890
Raymond M, Rousset F (1995) GENEPOP (V. 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rocha Filho LC, Cerântola NCM, Garófalo CA, Del Lama MA (2013) Genetic differentiation of the Euglossini (Hymenoptera, Apidae) population on a mainland coastal plain and an insular in Southeastern Brazil. Genetica 141:65–74
Roubik DW, Hanson PE (2004) Orchid bees of tropical America: biology and field guide. Heredia, Costa Rica
Roubik DW, Weight LA, Bonilla MA (1996) Population genetics, diploid males, and limits to social evolution of Euglossine bees. Evolution 50:931–935
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Schlötterer C (1998) Microsatellites. In: Hoelzel AR (ed) Molecular genetic analysis of populations. A practical approach. Oxford University Press, Oxford, pp 237–261
Schlötterer C (2004) The evolution of molecular markers—just a matter of fashion? Nat Rev Genet 5:63–69
Soro A, Field J, Bridge C, Cardinal SC, Paxton RJ (2010) Genetic differentiation across the social transition in a socially polymorphic sweat bee, Halictus rubicundus. Mol Ecol 19:3351–3363
Souza RO, Cervini MA, Del Lama MA, Paxton RJ (2007) Microsatellite loci for euglossine bees (Hymenoptera: Apidae). Mol Ecol Notes 7:1352–1356
Souza RO, Del Lama MA, Cervini M, Mortari N, Eltz T, Zimmermann Y, Bach C, Brosi BJ, Suni S, Quezada-Euán JJG, Paxton RJ (2010) Conservation genetics of neotropical pollinators revisited: microsatellite analysis suggests that diploid males are rare in orchid bees. Evolution 64:3318–3326
Spear SF, Balkenhol N, Fortin M-J, McRae BH, Scribner KIM (2010) Use of resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Mol Ecol 19:3576–3591
Spielman D, Brook BW, Frankham R (2004) Most species are not driven to extinction before genetic factors impact them. PNAS 101:15261–15264
Stouthamer R, Luck RF, Werren JH (1992) Genetics of sex determination and improvement of biological control using parasitoids. Environ Entomol 21:427–435
Suguio K, Martin L (1987) Classificação da costa e evolução geológica das planícies litorâneas do sudeste e sul do Brasil. In: Anáis do Simpósio sobre ecossistemas da Costa Sul e Sudeste Brasileira: síntese dos conhecimentos, pp 1–2
Suni SS, Brosi BJ (2011) Population genetics of orchid bees in a fragmented tropical landscape. Conserv Genet. doi:10.1007/s10592-011-0284-z
Takahashi J, Ayabe T, Mitsuhata M, Shimizu I, Ono M (2008) Diploid male production in a rare locally distributed bumblebee, Bombus florigelus (Hymenoptera, Apidae). Insect Soc 55:43–50
Tavares MG, Carvalho CR, Soares FAF (2010) Genome size variation in Melipona species (Hymenoptera: Apidae) and sub-grouping by their DNA content. Apidologie 41:636–642
Van Wilgenburg E, Driesses G, Beukboom LW (2006) Single locus complementary sex determination in Hymenoptera: an “unintelligent” design? Front Zool. doi:10.1186/1742-9994-3-1
Verhulst EC, Beukeboom LW, van de Zande L (2010) Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 328:620–623
Walsh PS, Metzeger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Whidden TL, Owen RE (2011) Frequencies of diploid male in natural population of three North American bumble bee (Bombus) species (Hymenoptera: Apidae). Genetics 104:83–87
Whitehorn PR, Tinsley MC, Goulson D (2009) Kin recognition and inbreeding reluctance in bumblebees. Apidologie 40:627–633
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420
Wright S (1969) Evolution and the genetics of populations. The theory of gene frequencies, vol 2. University of Chicago Press, Chicago
Zayed A (2009) Bee genetics and conservation. Apidologie 40:237–262
Zayed A, Packer L (2001) High levels of diploid male production in a primitively eusocial bee (Hymenoptera: Halictidae). Heredity 87:631–636
Zayed A, Packer L (2005) Complementary sex determination substantially increases extinction proneness of haplodiploid population. PNAS 102:10742–10746
Zayed A, Roubik DW, Packer L (2004) Use of diploid male frequency data as an indicator of pollinator decline. Proc R Soc Lond B 271:9–12
Zimmermann Y, Roubik DW, Quezada-Euan JJG, Paxton RJ, Eltz T (2009) Single mating in orchid bees (Euglossa, Apinae): implications for mate choice and social evolution. Insect Soc 56:241–249
Zimmermann Y, Schorkopf DLP, Moritz RFA, Pemberton RW, Quezada-Euan JJG, Eltz T (2011) Population genetic structure of orchid bees (Euglossini) in anthropogenically altered landscapes. Conserv Genet 12:1183–1194
Acknowledgments
We thank Carlos Eduardo Pinto, Guaraci Duran Cordeiro, Morgana Sazan, Paulo César Fernandes and Tiago Caetano for their help in the field, André Nemésio and Leo Rocha Filho for species identification, Flávio Oliveira Francisco, Leandro Rodrigues Santiago, Paulo Gonçalves and Rita Radzeviciute for help in the lab, and Maria Cristina Arias, Tomás Murray and the referees for many very helpful comments that helped improve the manuscript. Petra Liebe kindly processed samples in a MegaBace in the Molecular Ecology Lab at the Martin Luther University Halle-Wittenberg, for which we thank her and Robin Moritz. The Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) supported our project. The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Deutscher Akademischer Austausch Dienst (DAAD) funded the first author. SISBIO (Sistema de Informação e Autorização em Biodiversidade) provided a collecting permit (Nr.10453-1) and IBAMA (Insituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis) provided a biological sample export permit (Nr.10BR005548/DF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boff, S., Soro, A., Paxton, R.J. et al. Island isolation reduces genetic diversity and connectivity but does not significantly elevate diploid male production in a neotropical orchid bee. Conserv Genet 15, 1123–1135 (2014). https://doi.org/10.1007/s10592-014-0605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0605-0