Abstract
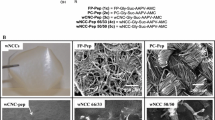
Here we describe the synthesis and characterization of peptide conjugated cellulose and nanocellulose materials as sensors for fluorescent detection of human neutrophil elastase (HNE). The cellulose sensor surfaces selected are filter paper (FP) and print cloth (PC) fabric, which are composed of processed cotton fibers. The nanocellulose based sensors have transducer surfaces comprised of cellulose nanocrystals (wCNC) and microfibrillated cellulose (MFC) derived from wood that are fabricated as wood nanocellulose composites (wNCCs) consisting of blended quantities of nanocrystalline and MFC at 66/33 and 50/50 ratios. These ratios were selected to examine the effect of significantly different CNC loadings had on surface area and peptide uptake. The fluorescent peptide HNE substrate, n-succinyl-Ala-Pro-Ala-4-amido-7-methylcoumarin (Pep) was attached to both cellulosic and nanocellulosic matrices and conjugated peptide analogs were confirmed by mass spectrometry (MS) and infrared (IR). The nanocellulose biosensors wCNC-Pep (3c) and wNCC-Pep (4c, 66/33 and 5c, 50/50) have higher levels of peptide incorporation than the cellulosic biosensors FP-Pep (1c) and PC-Pep (2c). The range of incorporation for the cellulosic sensors is 7–25 μg/mg and for the nanocellulose sensors 30–80 μg/mg. The degree of substitution of peptide was found to be in the order of approximate number of peptides per 200 anhydroglucose residues, 1 in PC-Pep (2c), 2 in FP-Pep (1c), 4 in wNCC-Pep (5c, 50/50), 6 in wNCC-Pep (4c, 66/33), and 12 in wCNC-Pep (3c). The specific surface areas of the sensors ranged from 0.016 to 261 m2 g−1 and correlated with degree of substitution of peptide on the cellulosic and nanocellulosic surfaces. Of the cellulose and nanocellulose biosensors, the wCNC-Pep (3c) has the highest level of peptide incorporation and the highest specific surface area, which makes it the preferred sensor matrix for human neutrophil elastase.





Similar content being viewed by others
References
Bellamy LJ (ed) (1975) Amides, proteins and polypeptides. In: The infrared spectra of complex molecules, vol 1. Chapman and Hall, London, pp 231–262
Blackwell HE (2006) Hitting the SPOT: small-molecule macroarrays advance combinatorial synthesis. Curr Opin Chem Biol 10:203–212. doi:10.1016/j.cbpa.2006.04.026
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. doi:10.1021/ja01269a023
Chan WC, White PD (2004) Fmoc solid phase peptide synthesis: a practical approach. Oxford University Press, Oxford
Chen R, Jakes KA (2002) Effect of pressing on the infrared spectra of single cotton fibers. Appl Spectrosc 56:646–650
Choi S, Goryll M, Sin L, Wong P, Chae J (2011) Microfluidic-based biosensors toward point-of-care detection of nucleic acids and proteins. Microfluid Nanofluid 10:231–247. doi:10.1007/s10404-010-0638-8
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr Polym 58:417–420. doi:10.1016/j.carbpol.2004.08.005
Dodson B (2012) Wood pulp extract stronger than carbon fiber or kevlar. http://www.gizmag.com/cellulose-nanocrystals-stronger-carbon-fiber-kevlar/23959/. Accessed 01 May 2015. 2015
Edwards JV, Prevost N, French AD, Concha M, DeLucca A, Wu Q (2013a) Nanocellulose-based biosensors: design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having fluorimetric and colorimetric elastase detection sensitivity. Engineering 5:20–28
Edwards JV, Prevost N, Sethumadhavan K, Ullah A, Condon B (2013b) Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 20:1223–1235. doi:10.1007/s10570-013-9901-y
Edwards JV, Prevost NT, French AD, Concha M, Condon BD (2015) Kinetic and structural analysis of fluorescent peptides on cotton cellulose nanocrystals as elastase sensors. Carbohydr Polym 116:278–285. doi:10.1016/j.carbpol.2014.04.067
Espino-Pérez E, Domenek S, Belgacem N, Sillard C, Bras J (2014) Green process for chemical functionalization of nanocellulose with carboxylic acids. Biomacromolecules 15:4551–4560. doi:10.1021/bm5013458
Eyley S, Thielemans W (2011) Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chem Commun 47:4177–4179. doi:10.1039/C0CC05359G
Fleming K, Gray DG, Matthews S (2001) Cellulose crystallites. Chem: A Eur J 7:1831–1836. doi:10.1002/1521-3765(20010504)7:9<1831:AID-CHEM1831>3.0.CO;2-S
Fontenot KR, Edwards JV, Haldane D, Graves E, Citron MS, Prevost NT, French AD, Condon BD (2016) Human neutrophil elastase detection with fluorescent peptide sensors conjugated to cellulosic and nanocellulosic materials: part II, structure/function analysis. Cellulose. doi:10.1007/s10570-016-0873-6
French A, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 20:583–588. doi:10.1007/s10570-012-9833-y
Gilbert C, Kokot S, Meyer U (1993) Application of DRIFT spectroscopy and chemometrics for the comparison of cotton fabrics. Appl Spectrosc 47:741–748
Gousse C, Chanzy H, Excoffier G, Soubeyrand L, Fleury E (2002) Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 43:2645–2651. doi:10.1016/S0032-3861(02)00051-4
Habibi Y, Chanzy H, Vignon M (2006) TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 13:679–687. doi:10.1007/s10570-006-9075-y
Kadla JF, Kubo S (2003) Miscibility and hydrogen bonding in blends of poly(ethylene oxide) and kraft lignin. Macromolecules 36:7803–7811. doi:10.1021/ma0348371
Kondo T, Sawatari C, Manley RSJ, Gray DG (1994) Characterization of hydrogen bonding in cellulose-synthetic polymer blend systems with regioselectively substituted methylcellulose. Macromolecules 27:210–215. doi:10.1021/ma00079a031
Kumar S et al (2013) Microfluidic-integrated biosensors: prospects for point-of-care diagnostics. Biotechnol J 8:1267–1279. doi:10.1002/biot.201200386
Lahiji RR, Reifenberger R, Moon RJ, Rudie A (2008) Characterization of cellulose nanocrystals by SPM. In: NSTI nanotechnology conference and trade show: life sciences, medicine and bio materials, Boston, Massachusetts, June 1–5, 2008. Nano Science and Technology Institute, Inc., Boca Raton, FL, USA, pp 704–707
Lam E, Male KB, Chong JH, Leung ACW, Luong JHT (2012) Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol 30:283–290. doi:10.1016/j.tibtech.2012.02.001
Lin N, Huang J, Dufresne A (2012) Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4:3274–3294. doi:10.1039/C2NR30260H
Lu J, Askeland P, Drzal LT (2008) Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 49:1285–1296. doi:10.1016/j.polymer.2008.01.028
Mariotti F, Tomé D, Mirand PP (2008) Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr 48:177–184. doi:10.1080/10408390701279749
Mark H, Gaylord N, Bikales N (2002) Degree of Substitution. In: Matyjaszewski K (ed) Encyclopedia of polymer science and technology. Wiley. doi:10.1002/0471440264.pst445
Missoum K, Belgacem MN, Barnes J-P, Brochier-Salon M-C, Bras J (2012) Nanofibrillated cellulose surface grafting in ionic liquid. Soft Matter 8:8338–8349. doi:10.1039/C2SM25691F
Mohanty SP, Kougianos E (2006) Biosensors: a tutorial review. IEEE Potentials 25:35–40. doi:10.1109/MP.2006.1649009
Nam S, French AD, Condon BD, Concha M (2016) Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr Polym 135:1–9. doi:10.1016/j.carbpol.2015.08.035
Orlandin A, Formaggio F, Toffoletti A, Peggion C (2014) Cotton functionalized with peptides: characterization and synthetic methods. J Pept Sci 20:547–553. doi:10.1002/psc.2659
Panthapulakkal S, Sain M (2012) Preparation and characterization of cellulose nanofibril films from wood fibre and their thermoplastic polycarbonate composites. Int J Polym Sci 2012:6. doi:10.1155/2012/381342
Peng Y, Gardner DJ, Han Y, Cai Z, Tshabalala MA (2013) Drying cellulose naocrystal suspensions. In: Postek MT, Moon RJ, Rudie AW, Bilodeau MA (eds) Production and applications of cellulose nanomaterials. TAPPI Press, Georgia, pp 31–33
Reimhult E, Höök F (2015) Design of surface modifications for nanoscale sensor applications. Sensors 15:1635–1675
Reiner RS, Rudie AW (2013) Process scale-up of cellulose nanocrystal production to 25 kg per batch at the forest products laboratory. In: Postek MT, Moon RJ, Rudie AW, Bilodeau MA (eds) Production and applications of cellulose nanomaterials. TAPPI Press Inc, Peachtree Corners, pp 21–24
Roeges NPG (1994a) A guide to the complete interpretation of infrared spectra of organic structures. Wiely, Chichester. doi:10.1021/ed072pA93.4
Roeges NPG (1994b) Normal vibrations and absorption regions of nitrogen compounds. A guide to the complete intrepretation of infrared spectra of organic structures. Wiley, Chichester, pp 231–239
Rowland SP, Howley PS (1988) Hydrogen bonding on accessible surfaces of cellulose from various sources and relationship to order within crystalline regions. J Polym Sci Part A: Polym Chem 26:1769–1778. doi:10.1002/pola.1988.080260708
Salas C, Nypelö T, Rodriguez-Abreu C, Carrillo C, Rojas OJ (2014) Nanocellulose properties and applications in colloids and interfaces. Curr Opin Colloid Interface Sci 19:383–396. doi:10.1016/j.cocis.2014.10.003
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. doi:10.1177/004051755902901003
Sehaqui H, Zhou Q, Ikkala O, Berglund LA (2011) Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 12:3638–3644. doi:10.1021/bm2008907
Sehaqui H, Zimmermann T, Tingaut P (2014) Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 21:367–382. doi:10.1007/s10570-013-0110-5
Sibrian-Vazquez M, Jensen TJ, Hammer RP, Vicente MGH (2006) Peptide-mediated cell transport of water soluble porphyrin conjugates. J Med Chem 49:1364–1372. doi:10.1021/jm050893b
Siqueira G, Bras J, Dufresne A (2010) New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 26:402–411. doi:10.1021/la9028595
Stadler A, Kappe CO (2001) The effect of microwave irradiation on carbodiimide-mediated esterifications on solid support. Tetrahedron 57:3915–3920. doi:10.1016/S0040-4020(01)00260-5
Tamayo J, Kosaka PM, Ruz JJ, San Paulo A, Calleja M (2013) Biosensors based on nanomechanical systems. Chem Soc Rev 42:1287–1311. doi:10.1039/C2CS35293A
Touzinsky GF, Gordon SM (1979) Degree of subsitution of cellulose derivatives containing n different substituent groups. Carbohydr Res 69:327–329. doi:10.1016/S0008-6215(00)85787-0
Trejo-O’Reilly J-A, Cavaille J-Y, Gandini A (1997) The surface chemical modification of cellulosic fibres in view of their use in composite materials. Cellulose 4:305–320. doi:10.1023/A:1018452310122
Wang J (2006) Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens Bioelectron 21:1887–1892. doi:10.1016/j.bios.2005.10.027
Wang S, Liu Q, Luo Z, Wen L, Cen K (2007) Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front Energy Power Eng China 1:413–419. doi:10.1007/s11708-007-0060-8
Xiong R, Han Y, Wang Y, Zhang W, Zhang X, Lu C (2014) Flexible, highly transparent and iridescent all-cellulose hybrid nanopaper with enhanced mechanical strength and writable surface. Carbohydr Polym 113:264–271. doi:10.1016/j.carbpol.2014.06.069
Xu X, Liu F, Jiang L, Zhu JY, Haagenson D, Wiesenborn DP (2013) Cellulose nanocrystals vs. cellulose nanofibrils: a comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl Mater Interfaces 5:2999–3009. doi:10.1021/am302624t
Acknowledgments
We thank Dr. Casey Grimm for mass spectral analysis and Zhongqi He and Dorselyn Chapital for allowing us to use their UV–VIS instrument.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, J.V., Fontenot, K.R., Haldane, D. et al. Human neutrophil elastase peptide sensors conjugated to cellulosic and nanocellulosic materials: part I, synthesis and characterization of fluorescent analogs. Cellulose 23, 1283–1295 (2016). https://doi.org/10.1007/s10570-016-0869-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0869-2