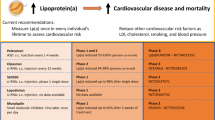
Abstract
In both epidemiologic and genetic studies, increased levels of Lp(a) have been associated with increased risk for cardiovascular diseases as well as aortic stenosis. However, until recently, it has been difficult to lower levels of Lp(a). Diet and lifestyle have little effect on plasma levels of Lp(a) which are mainly genetically determined. Emerging therapeutic agents which have recently become available, or which are undergoing clinical trials, can significantly lower Lp(a) levels. Studies with these agents will hopefully be able to provide more direct information whether reductions in Lp(a) will reduce CVD events independently of reduction in LDL-cholesterol levels.

Similar content being viewed by others
References
Boerwinkle E, Leffert C, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein (a) gene accounts for greater than 90 % of the variation in plasma lipoprotein (a) concentrations. J Clin Invest. 1992;90:52–60.
Rader DJ, Cain W, Ikewaki K, et al. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J Clin Invest. 1994;93:2758–63.
Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2012;125:241–49.
Heinrich J, Sandkamp M, Kokott R. Relationship of lipoprotein(a) to variables of coagulation and fibrinolysis in a healthy population. Clin Chem. 1991;37:1950–4.
Hoover-Plow J, Haung M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–91.
Howard BV, Roussouw JE. Estrogens and cardiovascular disease risk revisited: the Women’s Health Initiative. Curr Opin Lipidol. 2013;24:493–99.
Schlant RC, Forman S, Stamler J, Canner PL. The natural history of coronary heart disease: prognostic factors after recovery from myocardial infarction in 2789 men. the 5-year findings of the coronary drug project. Circulation. 1982;66(2):401–14.
Ledue TB, Neveux LM, Palomaki GE, Ritchie RF, Craig WY. The relationship between serum levels of lipoprotein (a) and proteins associated with acute phase response. Clin Chim Acta. 1993;223:73–82.
Maeda S, Abe A, Seishima M, Makino K, Noma A, Kawade M. Transient changes of serum lipoprotein(a) as an acute phase protein. Atherosclerosis. 1989;78:145–50.
Mbewu AD, Durrington PN, Bulleid S, et al. The immediate effect of streptokinase on serum lipoprotein (a) concentration and the effect of myocardial infarction on serum lipoprotein (a), apolipoprotein A1 and B, lipids and C-reactive protein. Atherosclerosis. 1993;103:65–71.
Gidding SS, Stone NJ, Bookstein LC, Laskarzewski PM, Stein EA. Month-to-month variability of lipids, lipoproteins, and apolipoproteins and the impact of acute infection in adolescents. J Pediatr. 1998;133:242–6.
Mooser V, Berger MM, Tappy L, Cayeux C, Markovina SM, Darioli R, et al. Major reduction in plasma Lp(a) levels during sepsis and burns. Arterioscler Thromb Vasc Biol. 2000;20:1137–42.
Noma A, Abe A, Maeda S. Lp(a): an acute-phase reactant? Chem Phys Lipids. 1994;67/68:411–17.
De Bruin T, van Barlingen H, van Linde-Sibenius TM, et al. Lipoprotein(a) and apolipoprotein B plasma concentrations in hypothyroid, euthyroid and hyperthyroid subjects. J Clin Endocrinol Metab. 1993;76:121–26.
Landerson PW, Kristensen JD, Ridgeway EC, et al. Use of the thyroid hormone analogue eprotirome in statin treated dyslipidemia. N Engl J Med. 2010;362:906–16.
Sjouke B, Langslet G, Ceska R, et al. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo controlled phase 3 study. Lancet Diabetes Endocrinol. 2014;2:455–63.
Krempler F, Kostner GM, Roscher A, et al. Studies on the role of specific cell surface receptors in the removal of lipoprotein(a) in man. J Clin Invest. 1983;71:1431–41.
Hofer G, Steyrer E, Kostner GM, Hermetter A. LDL-mediated interaction of Lp(a) with HepG2 cells: a novel fluorescence microscopy approach. J Lipid Res. 1997;38:2411–21.
Hafner S, Orchard T, Stein E, Schmidt D, LaBelle P. Effect of simvastatin on Lp(a) concentrations. Clin Cardiol. 1995;18:261–5.
Illingworth DR, Stein EA, Mitchel YB, Dujovne CA, Frost PH, Knopp RH, et al. Comparative effects of lovastatin and niacin in primary hypercholesterolemia: a prospective trial. Arch Intern Med. 1994;154:1586–95.
Hunninghake DB, Stein EA, Mellies MJ. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. J Clin Pharmacol. 1993;33(6):574–80.
Fieseler HG, Armstrong VW, Wieland E, Thiery J, Schutz E, Walli AK, et al. Serum Lp(a) concentrations are unaffected by treatment with the HMG-CoA reductase inhibitor Pravastatin: results of a 2-year investigation. Clin Chim Acta. 1991;204(1–3):291–300.
Gonbert S, Malinsky S, Sposito AC, Laouenan H, Doucet C, Chapman MJ, et al. Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis. 2002;164:305–11.
van Wissen S, Smilde TJ, Trip MD, de Boo T, Kastelein JJP, Stalenhoef AFH. Long term statin treatment reduces lipoprotein(a) concentrations in heterozygous familial hypercholesterolaemia. Heart. 2003;89:893–6.
Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the use of statins in prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129:635–42.
Berg K, Dahlen G, Christopherson B, et al. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin Genet. 1997;52:254–61.
Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–8.
Knopp RH, Gitter H, Truitt T. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;23:729–41.
Nozue T, Michishati I, Mizuguchi I. Effects of ezetimibe on remnant-like particle cholesterol, lipoprotein(a) and oxidized low-density lipoprotein in patients with dyslipidaemia. J Atheroscler Thromb. 2010;17(1):37–44.
Gagne C, Gaudet D, Bruckert E, et al. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–75.
Moutzouri E, Liberopoulos E, Mikhailidis DP, et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. Int J Clin Pract. 2011;65:1141–8.
Chennamsetty I, Claudel T, Kostner KM, et al. Farsenoid X receptor represses hepatic human apo(a) gene expression. J Clin Invest. 2011;121:3724–34.
Gurakar K, Hoeg JM, Kostner G, Papadopolous NM. Brewer HBJr, levels of lipoprotein (a) Lp(a) decline with neomycin and niacin treatment. Atherosclerosis. 1985;57:293–301.
Carlson LA, Hamsten A, Asplund A. Pronounced lowering of levels of lipoprotein (a) Lp(a) in hyperlipidemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–6.
Stein EA, Davidson MH, Dujovne CA, Hunninghake DB, et al. Efficacy and tolerability of low dose simvastatin and niacin, alone and in combination, in patients with combined hyperlipidemia: a prospective trial. J Cardiovasc Pharmacol Ther. 1996;1:107–16.
Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, et al. Sheehan JP; Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002;162(14):1568–76.
Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA. Brody. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82(12A):74U–81U.
Goldberg AC. Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study. Am J Cardiol. 1998;82(12A):35U–8U.
Sposito AC, Mansur AP, Maranhão RC, Rodrigues-Sobrinho CRM, Coelho OR, Ramires JAF. Etofibrate but not controlled-release niacin decreases LDL cholesterol and lipoprotein (a) in type IIb dyslipidemic subjects. Braz J Med Biol Res. 2001;34:177–82.
Shearer GC, Pottala JV, Hansen SN, Brandenburg V, Harris WS. Effects of prescription niacin and omega-3 fatty acids on lipids and vascular function in metabolic syndrome: a randomized controlled trial[S]. J Lipid Res. 2012;53:2429–35.
Visser ME, Witztum JL, Stroes ES, Kastelein JJ. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J. 2012;33:1451–58.
Santos RD, Raal FJ, Catapano A, et al. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol. 2015;35:689–99.
Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36:566–75.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of lower LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006.
Samaha FF, McKenney J, Bloedon LT, Sasiela WJ, Rader DJ. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2008;5:497–505.
Cuchel M, Meagher EA, du Toit TH, Blom DJ, Marais AD, Hegele RA, et al. Phase 3 HoFH Lomitapide Study Investigators. efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6.
Thompson GR, Barbir M, Davies D, Dobral P, Gesinde M, Livingston M, et al. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis. 2010;208:317–21.
Gordon BR, Kelsey SF, Bilheimer DW, Brown DC, Dau PC, Gotto AM, et al. For the Liposorber Study Group, treatment of refractory familial hypercholesterolemia by low-density lipoprotein apheresis using an automated dextran sulfate cellulose absorption system. Am J Cardiol. 1992;70:1010–6.
Leebmann J, Roeseler E, Julius U, Heigl F, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128:2567–76.
Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, et al. For the DEFINE Investigators Safety of anacetrapib in patients with, or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15.
Hovingh GK, Kastelein JJP, van Deventer SJH, Round P, Ford J, Saleheen D, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015.
Nicholls SJ, Ruotolo G, Brewer BH, Wang MD, Liu L, Willey MB, et al. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol. 2015. doi:10.1016/j.jacl.2015.11.014.
HPS3/TIMI55 – REVEAL trial of Anacetrapib in high-risk vascular patients recruits the target of 30,000 participantshttps://www.ctsu.ox.ac.uk/research/mega-trials/hps3-reveal [accessed January 10, 2016].
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Lilly to discontinue development of evacetrapib for high-risk atherosclerotic cardiovascular disease. https://investor.lilly.com/releasedetail.cfm?ReleaseID=936130 [accessed January 12, 2016].
Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18.
Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, et al. Reduction in lipoprotein (a) with the PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of over 1300 patients in 4 phase 2 trials. J Am Coll Cardiol. 2014;63(13):1278–88.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Kastelein JJ, Robinson JG, Farnier M, Krempf M, Langslet G, Lorenzato C, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia not adequately controlled with current lipid-lowering therapy: design and rationale of the ODYSSEY FH studies. Cardiovasc Drugs Ther. 2014;28(3):281–9.
Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, et al. Donahue S Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–46.
McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand A-C, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53.
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. New Engl J Med. 2015;372:1500–9.
Graham MJ, Viney N, Crooke R, Tsimikas S. Antisense inhibition of apolipoprotein(a) to lower plasma lipoprotein(a) levels in humans. J Lipid Res. 2015 Nov 4.
Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy 674 targeting apolipoprotein(a): a randomised, double-blind, placebo- 675 controlled phase 1 study. Lancet. 2015;386:1472–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Stein has received consulting fees from Amgen, Regeneron, Sanofi, Genentech/Roche, CymaBay, Gemphire, Catabasis, CVS/Caremark and BMS.
Dr. Raal has received reimbursement for conducting clinical trials from Amgen and Sanofi; modest speaker fees from AstraZeneca, Pfizer, and Merck; honoraria from AstraZeneca, Pfizer, Merck, Amgen, and Sanofi; and consultant/advisory board fees from AstraZeneca, Pfizer, and Merck.
Rights and permissions
About this article
Cite this article
Stein, E.A., Raal, F. Future Directions to Establish Lipoprotein(a) as a Treatment for Atherosclerotic Cardiovascular Disease. Cardiovasc Drugs Ther 30, 101–108 (2016). https://doi.org/10.1007/s10557-016-6654-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6654-5